Spino-cerebral Plasticity and Integrated Neuroimaging in Disease and Learning Laboratory
Principal Investigator: Julien Doyon, PhD, FRSC, FCAHS
Publications: PubMed, Google Scholar

Julien Doyon (pictured here in the center), PhD, FRSC, FCAHS is Director of the McConnell Brain Imaging Centre since October 2017. In the past, he has held several leadership roles related to the field of neuroimaging, including most recently as Scientific Director of the Unité de Neuroimagerie Fonctionnelle at the Centre de recherche, Institut universitaire de gériatrie de Montréal (2010-2018), Director of the Quebec Bio-Imaging Network (2008-2019), and codirector of the Laboratoire international de neuroimagerie et modélisation, INSERM‐ Université de Montréal 2007-2014.
Julien obtained his BA and his Masters in Psychology from the “Université Laval” in Quebec city, and completed his PhD in psychology in 1988 at McGill University under the supervision of Dr. Brenda Milner, a world renown pioneer in neuropsychology. Following training, he built a very successful scientific career in cognitive neuroscience.
Julien has received a number of prizes and awards in recognition of his contributions to the advancement of knowledge and leadership in the field of cognitive neuroscience and neuroimaging. He was presented the 2011 Canadian Society for Brain Behavior & Cognitive Sciences (CSBBCS) – Richard C. Tess Award and was named a Fellow of this society in 2017. In 2012, he was awarded the ACFAS ‐ Léo Pariseau prize highlighting the excellence and international impact of his work, and was elected as a Fellow of the Royal Society of Canada in June 2017 and Fellow of the Canadian Academy of Health Sciences in September 2018.
Overview of the research conducted in the lab
Research in our laboratory is structured around two poles: a fundamental research pole that is focused on the neuroscience of learning and memory and an applied research pole that seeks to apply the knowledge gained therein in neurodegenerative and neurological diseases, such as Parkinson’s and amyotrophic lateral sclerosis. In our basic research work, we use multi-modal imaging techniques (MRI, EEG) and multi-disciplinary approaches to investigate the way people acquire motor skills, and to characterize the brain and spinal cord neuroplasticity specific to motor learning at different time scales.
We also investigate the role of sleep in the consolidation of both procedural (i.e. motor skills) and declarative (i.e. information about facts and events) memories and the neurophysiological mechanisms underlying this phenomenon. In our applied research work, we seek to understand how aging and disease impact the cerebro-spinal neuroplasticity associated with motor learning and we use state-of-the-art neuroimaging techniques that we pioneered in the last 7 years to develop cerebro-spinal biomarkers of Parkinson’s disease that could be used for early screening and diagnosis, as well as for disease progression monitoring.
Major accomplishments
Scientific Accomplishments
Development of an influential model of neuroplasticity mediating motor skill learning
A key accomplishment of our laboratory has been the characterization of neural plasticity associated with motor skill learning and the development of an influential model in the field (over 2200 citations) describing the short- and long-term cerebral functional changes underlying different motor learning phases when acquiring different types of motor skills.
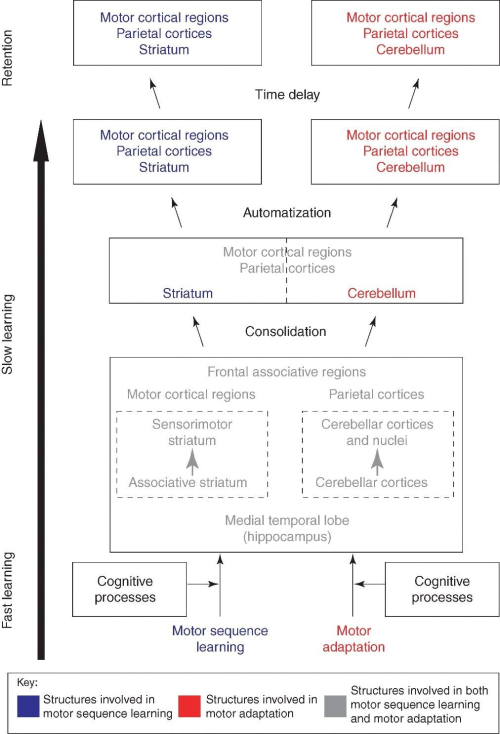
In humans, motor skill learning has been investigated using paradigms that assess either the ability to combine single movements into a smooth sequence (motor sequence learning [MSL]), or the ability to adjust motor responses to compensate for changing environmental conditions (motor adaptation [MA]). MSL paradigms typically require subjects to produce a motor sequence that they either know explicitly before training or learn implicitly through repeated practice in response to visual stimuli, whereas MA is often tested using kinematic tasks in which participants are exposed to visuomotor distortions. In both cases, behavioral performance is known to evolve incrementally following a series of stages: 1) an early within-session learning phase where the magnitude of behavioral improvement is substantial, and 2) a late across-session stage, in which a smaller, but constant amelioration in performance can be observed with extended practice. Based on animal and human research, several brain structures, including the striatum, cerebellum, and motor-related cortical regions of the frontal and parietal lobes (forming the cortico-striatal [CS] and cortico-cerebellar [CC] loops) have shown to be critical for the acquisition of these motor skills. The major advancement brought upon by our work was the development of a model parsing out the distinct contribution from each system during acquisition of different skills (i.e. MSL vs. MA). Specifically, we showed that where an interaction between the CS and CC is essential early in the acquisition process to create the optimal motor routine necessary to learn the task at hand, in the later phases, when the skill is well learned, a dissociation emerge between them. The CS neuroplasticity is underlying further improvement in performance on tasks requiring MSL, while that seen in CC plays a critical role in improving performance on skills that demand MA (Figure 1).
Development of a pioneering simultaneous brain-spinal cord neuroimaging technique and its application in motor learning research
In the past 7 years, our team and its collaborators has pioneered techniques that allow the simultaneous acquisition of functional magnetic resonance imaging (fMRI) data of the brain and cervical spinal cord (CSC) to investigate the latter’s contributions to motor learning. Using such an approach, we have shown that motor sequence learning (MSL) relies not only on brain functional plasticity, but also on intrinsic learning-dependent activity changes in the C6-C8 spinal segments, in conjunction with specific patterns of brain-CSC functional connectivity (Fig. 2A). Complementing our innovative simultaneous brain-CSC neuroimaging technique with electromyographic (EMG) recordings, we have further revealed that such plastic changes are related to specific neurophysiological processes that are known to reflect corticospinal modulations of local CSC excitability, such as the presynaptic and reciprocal inhibition mechanisms (Fig. 2B). Importantly, we have also replicated our imaging findings by showing the same intrinsic MSL-related plasticity in the CSC when using a motor task that involved different effectors (forearm vs. fingers), and have expanded our investigation by exploring the brain-CSC interaction in later phases of motor learning (i.e. after a week-long practice) (Fig. 2C). Finally, we have carried out the first characterization of the brain and CSC functional connectivity during rest, and the first comparison of resting-state activity before and after MSL, hence mapping the plasticity of the CSC using functional connectivity metrics based on an data-driven approach (Fig. 2D-F).
Finally, the development of the open-source Spinal Cord Toolbox (SCT) software by one of our former postdoctoral fellows and current collaborator (Dr. de Leener), currently provides researchers with access to anatomical templates and data analysis pipelines for the CSC, similar to those used for the brain. Therefore, while spinal neuroimaging still poses specific challenges due to the relatively small size of the CSC and inhomogeneity induced in the magnetic field by the surrounding structures, these state-of-the-art methodological advances offer researchers the opportunity to account for the contribution of this often neglected part of the CNS in a variety of experimental paradigms that focused exclusively on the brain, to date. Thus given the now overwhelming neuroimaging evidence provided by our research group that the brain and CSC are functionally well interconnected during motor learning tasks (Fig. 2A and 2C) and at rest (Fig. 2D and 2E), it has become imperative to investigate further the role of CSC and its interaction with the brain in motor learning, and to determine whether it generalizes to other forms of motor skills, acquisition processes and learning stages.

Neurophysiological mechanisms underlying sleep-related memory consolidation
Memories are a fundamental part of our identity, and thus understanding the mechanisms of how they are formed, maintained and enhanced is crucial as they guide our behavior in everyday life. One such mechanism that has attracted much attention is the memory consolidation, a process whereby freshly encoded mnemonic traces become more stable over time. Up to now, ample evidence is showing that both declarative and procedural types of memories can benefit from sleep, and spindle activity (bursts of 10–16 Hz oscillatory activity, < 2s.) generated during non-rapid eye movement (NREM) sleep. For instance, work in our laboratory has provided insights into the functional changes in brain activity, as well as sleep characteristics mediating procedural memory consolidation using a multimodal neuroimaging approach with fMRI and EEG acquired alone or simultaneously during sleep, as well as innovative data-driven large-scale network analyses. Our findings have revealed that: 1) MSL off-line gains (i.e. improvement in performance without additional practice, an indicator of motor memory consolidation) are associated with spindle activity during the post-training night (Fig. 3A), 2) MSL consolidation is enhanced when using a targeted memory reactivation paradigm where task-conditioned olfactory cues are presented during NREM-stage 2 sleep (but not REM) (Fig. 3B), 3) the cortico-striatal system as an integrated network, and the putamen in particular, are involved in the off-line, sleep-dependent, MSL consolidation process (Fig. 3C).

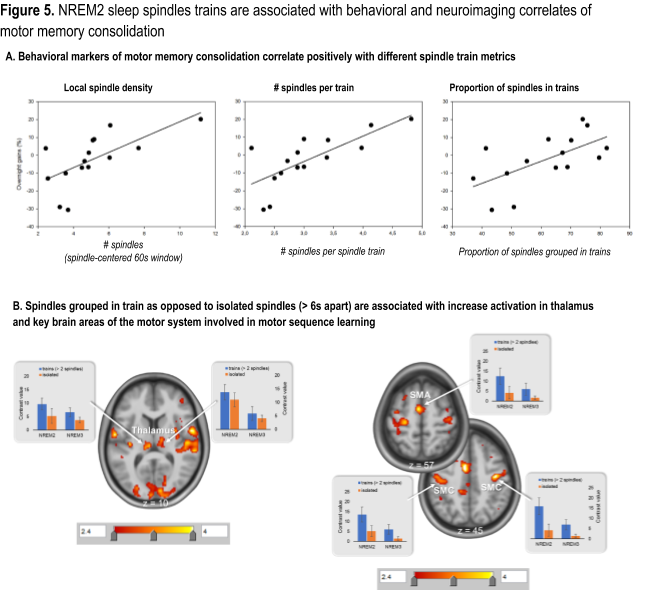
Yet our current state of knowledge does not provide a unitary view as to the nature of the sleep oscillatory patterns that are critical for memory consolidation, nor does it offer a clear answer as to the neural substrates that underlie this process for each memory type. To fill these knowledge gaps, we have recently developed metrics sensitive to spindle clustering and rhythmicity based on data from an electroencephalography – functional magnetic resonance imaging (EEG-fMRI) study examining the neural substrate of motor memory consolidation (MMC) using a procedural learning task (Boutin et al., 2018). Our results revealed that: (1) MMC is associated with increased coherence in EEG activity, time-locked to spindles, between cortical and subcortical brain structures known to also be involved in learning (Fig 4A and 4B) and (2) this increase was specific to spindles that are grouped in ‘trains’ (i.e. occurring less than 6 s apart), as opposed to isolated spindles (i.e. separated by more than 6 s) (Fig. 4C).

Furthermore, our most recent work in this area has demonstrated that behavioral markers of MMC correlated with spindle train metrics (Fig. 5A) and that spindle trains were also associated with greater activation in key learning-related brain regions (Fig. 5B).
Based on this data, our past work, and recently collected pilot data showing that the declarative memory consolidation is also associated with similar spindle metrics, our laboratory has recently obtained CIHR funding for a series of studies in which we propose to test a model explaining the similarities and differences in sleep-related consolidation of procedural and declarative memories. Our model postulates that the sleep reactivation/reprocessing of both forms of memories is facilitated by NREM spindles occurring in trains, as opposed to isolated ones, and that the rhythmic pattern of spindle trains results in increased functional connectivity within brain networks overlapping with those involved in the initial encoding phase. Thus, we posit that similar basic mechanism underlies the consolidation of both procedural and declarative memories (i.e. based on spindle trains), with the difference being only in its neural substrate, that is, the brain networks that are spontaneously reactivated during these spindle events.
Scientific productivity
Over the years, Dr. Doyon and his team published over 200 scientific papers, several of them in highly rated journals like Nature Neuroscience, PLoS Biology, and PNAS. Among the most important publications are also 11 chapters and an atlas (book) of the cerebellum in Talairach stereotaxic space that is widely used by the neuroimaging community. All lab members have contributed significantly to knowledge dissemination through more than 400 scientific communications and over 140 keynote lectures at national and international scientific meetings.
Training and mentorship
Over the years, Dr. Doyon has supervised, mentored and trained 15 master and 24 PhD students (3 currently), as well as 23 post-doctoral fellows (2 currently), 20 of whom are presently pursuing very successful academic careers in Quebec, Canada or abroad. A significant number of former lab members are now scientists leading their own laboratory and they continue to collaborate with the SPINDL Lab in various national and international projects.
Ongoing Projects
Currently, member of the SPINDL Lab are involved in 12 projects in various states of progress (5 in which data collection is completed, 4 for which data collection is ongoing and 3 that are in advanced stages of obtaining their IRB certificate). The major ongoing projects in our lab are:
- Spinal cord contribution to motor skill learning (funded by Curtois Foundation, NSERC)
- Identifying functional and structural MRI profiles specific to Parkinson’s disease (funded by Curtois Foundation, NSERC)
- Effect of sensory training on motor learning and spinal sensorimotor loops excitability (funded by NSERC)
- Consolidation and enhancement of procedural and declarative memories during sleep: uncovering age-related differences, neurophysiological mechanisms and developing new interventions (funded by Healthy Brains for Healthy Life)
- Characterizing and comparing sleep-related consolidation neurophysiological mechanisms for procedural and declarative memories (funded by CIHR)
Funding
Over the years, Dr. Doyon has been awarded over $10M in funding as principal investigator from provincial, national and international agencies (FRQS, Fondation Courtois, NSERC, CIHR, ERA-NET, HBHL, JPND), as well as over $15M as co-principal investigator (co-PI). As co-director of the “Regroupement provincial de recherche en imagerie cérébrale” (REPRIC) and director of the Quebec Bio-Imaging Network (QBIN), he has secured over $4.3M in institutional grants. He has also received $3.1M as principal investigator in three Canada Foundation for Innovation (CFI) grants, as well as $39.2M in three other CFI grants, as co-PI. Finally, under his leadership, since the start of his tenure as director, the McConnell Brain Imaging Center has received $9.8M in institutional grants.
Lab Members
The current composition of the SPINDL Lab team includes two academic associates (Ovidiu Lungu, PhD; Ella Gabitov, PhD), two research assistants (Latifa Lazzouni, PhD and Thibault Vlieghe, Eng., MSc), two post-doctoral fellows (Caroline Landelle, PhD; Bilal Alchalabi, PhD) and three PhD students (Shuo Chen, MSc; Vaishali Mutreja and Prakriti Gupta). Details about each member of the lab can be found here.
Details about each lab member
|
Ovidiu Lungu, PhD; Academic Associate
|
Education:
|
|
Ella Gabitov, PhD; Academic Associate
|
Education:
|
|
Latifa Lazzouni, PhD; Research Assistant
|
Education:
|
|
Thibault Vlieghe, Eng. MSc; Research Assistant
|
Education:
|
|
Caroline Landelle, PhD; Postdoctoral Fellow
|
Education:
|
|
Bilal Alchalabi, PhD; Postdoctoral Fellow
|
Education:
|
|
Shuo Chen, PhD student
|
Education:
|
|
Vaishali Mutreja, PhD student
|
Education:
|
|
Prakriti Gupta, PhD student
|
Education:
|
Collaborations
McGill University, Montréal, CANADA
- Robert Zatorre
- Edward Fon
- Madeleine Sharp
- Leslie Fellows
- Birgit Frauscher
Université de Montréal, Montréal, CANADA
- Julie Carrier
Concordia University, Montréal, CANADA
- Habib Benali
Polytechnique Montréal, Montréal, CANADA
- Benjamin de Leener
- Julien Cohen-Adad
École de technologie supérieure, Montréal, CANADA
- Jean-Marc Lina
Katholieke Universiteit, Leuven, BELGIUM
- Genevieve Albouy
- Bradley King
Sorbonne Université, Paris, FRANCE
- Veronique Marchand-Pauvert
- Pierre-François Pradat
Brain and Spine Institute (ICM), Paris, FRANCE
- Isabelle Le Ber
Université Paris-Saclay, Paris, FRANCE
- Arnaud Boutin
University of Florida, Gainsville, USA
- Shahab Vahdat
University of Birmingham, Birmingham, UK
- Ali Khatibi
University College of London, London, UK
- Daniel Alexander
- Gary Zhang
Haifa University, Haifa, ISRAEL
- Avi Karni
- Maria Korman
Sidney University, Sidney, AUSTRALIA
- Mathew Kiernan
Publications
Over the years, Dr. Doyon and his team published over 200 scientific papers, several of them in highly rated journals like Nature Neuroscience, PLoS Biology, and PNAS. We present here a list of selected publications.
Selected Publications
Here are some of our selected publications.
- Vahdat S, Khatibi A, Lungu O, Finsterbusch J, Büchel C, Cohen-Adad J, Marchand-Pauvert V, Doyon J. Resting-state brain and spinal cord networks in humans are functionally integrated. PLoS Biol. 2020 Jul 2;18(7):e3000789. doi: 10.1371/journal.pbio.3000789. PMID: 32614823; PMCID: PMC7363111.
- Solstrand Dahlberg L, Lungu O, Doyon J. Cerebellar Contribution to Motor and Non-motor Functions in Parkinson's Disease: A Meta-Analysis of fMRI Findings. Front Neurol. 2020 Feb 27;11:127. doi: 10.3389/fneur.2020.00127. PMID: 32174883; PMCID: PMC7056869.
- Boutin A, Doyon J. A sleep spindle framework for motor memory consolidation. Philos Trans R Soc Lond B Biol Sci. 2020 May 25;375(1799):20190232. doi: 10.1098/rstb.2019.0232. Epub 2020 Apr 6. PMID: 32248783; PMCID: PMC7209914.
- Gabitov E, Lungu O, Albouy G, Doyon J. Weaker Inter-hemispheric and Local Functional Connectivity of the Somatomotor Cortex During a Motor Skill Acquisition Is Associated With Better Learning. Front Neurol. 2019 Nov 27;10:1242. doi: 10.3389/fneur.2019.01242. PMID: 31827459; PMCID: PMC6890719.
- Pinsard B, Boutin A, Gabitov E, Lungu O, Benali H, Doyon J. Consolidation alters motor sequence-specific distributed representations. Elife. 2019 Mar 18;8:e39324. doi: 10.7554/eLife.39324. PMID: 30882348; PMCID: PMC6461441.
- Gabitov E, Boutin A, Pinsard B, Censor N, Fogel SM, Albouy G, King BR, Carrier J, Cohen LG, Karni A, Doyon J. Susceptibility of consolidated procedural memory to interference is independent of its active task-based retrieval. PLoS One. 2019 Jan 17;14(1):e0210876. doi: 10.1371/journal.pone.0210876. PMID: 30653576; PMCID: PMC6336251.
- Boutin A, Pinsard B, Boré A, Carrier J, Fogel SM, Doyon J. Transient synchronization of hippocampo-striato-thalamo-cortical networks during sleep spindle oscillations induces motor memory consolidation. Neuroimage. 2018 Apr 1;169:419-430. doi: 10.1016/j.neuroimage.2017.12.066. Epub 2017 Dec 24. PMID: 29277652.
- Vahdat S, Fogel S, Benali H, Doyon J. Network-wide reorganization of procedural memory during NREM sleep revealed by fMRI. Elife. 2017 Sep 11;6:e24987. doi: 10.7554/eLife.24987. PMID: 28892464; PMCID: PMC5593513.
- Fogel S, Albouy G, King BR, Lungu O, Vien C, Bore A, Pinsard B, Benali H, Carrier J, Doyon J. Reactivation or transformation? Motor memory consolidation associated with cerebral activation time-locked to sleep spindles. PLoS One. 2017 Apr 19;12(4):e0174755. doi: 10.1371/journal.pone.0174755. PMID: 28422976; PMCID: PMC5396873.
- Fogel S, Vien C, Karni A, Benali H, Carrier J, Doyon J. Sleep spindles: a physiological marker of age-related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol Aging. 2017 Jan;49:154-164. doi: 10.1016/j.neurobiolaging.2016.10.009. Epub 2016 Oct 11. PMID: 27815989.
- Duchesne C, Gheysen F, Bore A, Albouy G, Nadeau A, Robillard ME, Bobeuf F, Lafontaine AL, Lungu O, Bherer L, Doyon J. Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson's disease individuals. Neuroimage Clin. 2016 Sep 14;12:559-569. doi: 10.1016/j.nicl.2016.09.011. PMID: 27689020; PMCID: PMC5031470.
- Laventure S, Fogel S, Lungu O, Albouy G, Sévigny-Dupont P, Vien C, Sayour C, Carrier J, Benali H, Doyon J. NREM2 and Sleep Spindles Are Instrumental to the Consolidation of Motor Sequence Memories. PLoS Biol. 2016 Mar 31;14(3):e1002429. doi: 10.1371/journal.pbio.1002429. PMID: 27032084; PMCID: PMC4816304.
- King BR, Saucier P, Albouy G, Fogel SM, Rumpf JJ, Klann J, Buccino G, Binkofski F, Classen J, Karni A, Doyon J. Cerebral Activation During Initial Motor Learning Forecasts Subsequent Sleep-Facilitated Memory Consolidation in Older Adults. Cereb Cortex. 2017 Feb 1;27(2):1588-1601. doi: 10.1093/cercor/bhv347. PMID: 26802074.
- Duchesne C, Lungu O, Nadeau A, Robillard ME, Boré A, Bobeuf F, Lafontaine AL, Gheysen F, Bherer L, Doyon J. Enhancing both motor and cognitive functioning in Parkinson's disease: Aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015 Oct;99:68-77. doi: 10.1016/j.bandc.2015.07.005. Epub 2015 Aug 7. PMID: 26263381.
- Albouy G, Fogel S, King BR, Laventure S, Benali H, Karni A, Carrier J, Robertson EM, Doyon J. Maintaining vs. enhancing motor sequence memories: respective roles of striatal and hippocampal systems. Neuroimage. 2015 Mar;108:423-34. doi: 10.1016/j.neuroimage.2014.12.049. Epub 2014 Dec 23. PMID: 25542533.
- Lungu O, Monchi O, Albouy G, Jubault T, Ballarin E, Burnod Y, Doyon J. Striatal and hippocampal involvement in motor sequence chunking depends on the learning strategy. PLoS One. 2014 Aug 22;9(8):e103885. doi: 10.1371/journal.pone.0103885. PMID: 25148078; PMCID: PMC4141721.
- Debas K, Carrier J, Barakat M, Marrelec G, Bellec P, Hadj Tahar A, Karni A, Ungerleider LG, Benali H, Doyon J. Off-line consolidation of motor sequence learning results in greater integration within a cortico-striatal functional network. Neuroimage. 2014 Oct 1;99:50-8. doi: 10.1016/j.neuroimage.2014.05.022. Epub 2014 May 17. PMID: 24844748; PMCID: PMC6644011.
- Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, Jbabdi S, Benali H, Karni A, Maquet P, Carrier J, Doyon J. fMRI and sleep correlates of the age- related impairment in motor memory consolidation. Hum Brain Mapp. 2014 Aug;35(8):3625-45. doi: 10.1002/hbm.22426. Epub 2013 Dec 2. PMID: 24302373; PMCID: PMC6869653.
- Albouy G, King BR, Maquet P, Doyon J. Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus. 2013 Nov;23(11):985-1004. doi: 10.1002/hipo.22183. PMID: 23929594.
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, Carrier J, Robertson E, Doyon J. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One. 2013;8(1):e52805. doi: 10.1371/journal.pone.0052805. Epub 2013 Jan 2. PMID: 23300993; PMCID: PMC3534707.
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17839-44. doi: 10.1073/pnas.1013176107. Epub 2010 Sep 27. PMID: 20876115; PMCID: PMC2955095.
- Lungu O, Frigon A, Piché M, Rainville P, Rossignol S, Doyon J. Changes in spinal reflex excitability associated with motor sequence learning. J Neurophysiol. 2010 May;103(5):2675-83. doi: 10.1152/jn.00006.2010. Epub 2010 Mar 17. PMID: 20237314.
- Orban P, Peigneux P, Lungu O, Albouy G, Breton E, Laberenne F, Benali H, Maquet P, Doyon J. The multifaceted nature of the relationship between performance and brain activity in motor sequence learning. Neuroimage. 2010 Jan 1;49(1):694-702. doi: 10.1016/j.neuroimage.2009.08.055. Epub 2009 Sep 2. PMID: 19732838.
- Orban P, Lungu O, Doyon J. Motor sequence learning and developmental dyslexia. Ann N Y Acad Sci. 2008 Dec;1145:151-72. doi: 10.1196/annals.1416.016. PMID: 19076395.
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009 Apr 12;199(1):61-75. doi: 10.1016/j.bbr.2008.11.012. Epub 2008 Nov 17. PMID: 19061920.
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008 Aug;21(4):478-83. doi: 10.1097/WCO.0b013e328304b6a3. PMID: 18607210.
- Marrelec G, Bellec P, Krainik A, Duffau H, Pélégrini-Issac M, Lehéricy S, Benali H, Doyon J. Regions, systems, and the brain: hierarchical measures of functional integration in fMRI. Med Image Anal. 2008 Aug;12(4):484-96. doi: 10.1016/j.media.2008.02.002. Epub 2008 Feb 15. PMID: 18396441.
- Marrelec G, Horwitz B, Kim J, Pélégrini-Issac M, Benali H, Doyon J. Using partial correlation to enhance structural equation modeling of functional MRI data. Magn Reson Imaging. 2007 Oct;25(8):1181-9. doi: 10.1016/j.mri.2007.02.012. Epub 2007 May 1. PMID: 17475433.
- Perlbarg V, Bellec P, Anton JL, Pélégrini-Issac M, Doyon J, Benali H. CORSICA: correction of structured noise in fMRI by automatic identification of ICA components. Magn Reson Imaging. 2007 Jan;25(1):35-46. doi: 10.1016/j.mri.2006.09.042. Epub 2006 Nov 30. PMID: 17222713.
- Marrelec G, Krainik A, Duffau H, Pélégrini-Issac M, Lehéricy S, Doyon J, Benali H. Partial correlation for functional brain interactivity investigation in functional MRI. Neuroimage. 2006 Aug 1;32(1):228-37. doi: 10.1016/j.neuroimage.2005.12.057. Epub 2006 Jun 13. PMID: 16777436.
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006 Feb;59(2):257-64. doi: 10.1002/ana.20742. PMID: 16437582.
- Bellec P, Perlbarg V, Jbabdi S, Pélégrini-Issac M, Anton JL, Doyon J, Benali H. Identification of large-scale networks in the brain using fMRI. Neuroimage. 2006 Feb 15;29(4):1231-43. doi: 10.1016/j.neuroimage.2005.08.044. Epub 2005 Oct 24. PMID: 16246590.
- Lehéricy S, Benali H, Van de Moortele PF, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12566-71. doi: 10.1073/pnas.0502762102. Epub 2005 Aug 17. PMID: 16107540; PMCID: PMC1194910.
- Penhune VB, Doyon J. Cerebellum and M1 interaction during early learning of timed motor sequences. Neuroimage. 2005 Jul 1;26(3):801-12. doi: 10.1016/j.neuroimage.2005.02.041. Epub 2005 Apr 7. PMID: 15955490.
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005 Apr;15(2):161-7. doi: 10.1016/j.conb.2005.03.004. PMID: 15831397.
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002 Nov;78(3):553-64. doi:10.1006/nlme.2002.4091. PMID: 12559834.
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico- striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252-62. doi: 10.1016/s0028-3932(02)00158-6. PMID: 12457751.













