Current research in the Blum Group
Self-assembly on a Tobacco mosaic virus coat protein template

While current nanoscale production is dominated by top-down methods such as ion, e-beam, or photo- lithography, the significant increase in cost involved as feature sizes decrease below 50 nm makes alternative techniques more attractive. Furthermore, it has beome clear that producing nano-based materials with properties not found in nature requires breakthroughs in the ability to position materials with nanometer precision. Overcoming these obstacles has led to a growing interest in bottom-up, self-assembling systems. One approach is to use biomolecules as scaffolds because of the specificity and versatility they provide. In particular, the use of viruses as nanoscale scaffolds offers the promise of exquisite control for positioning on the nanoscale, using a particle that can undergo further self-assembly into extended structures, and allowing the simultaneous creation of many identical complex submicron geometrical structures.
As platforms for templated self-assembly, plant viruses have the advantage of having no pathogenicity towards humans and can often be utilized without their infectious genome. This is because most of the chemical functionality that assembles the capsid is incorporated into the proteins themselves. As a result, they can often assemble independently or around a simulant of the genetic material. These Virus-Like Particles (VLPs) assemble into a variety of geometries and can also respond to chemical and physical changes in their environment such as pH and temperature. In the Blum Group, we use Tobacco mosaic virus coat protein as a scaffold for self-assembly.
Controlling the composition and surface chemistry of superparamagnetic iron oxide nanoparticles
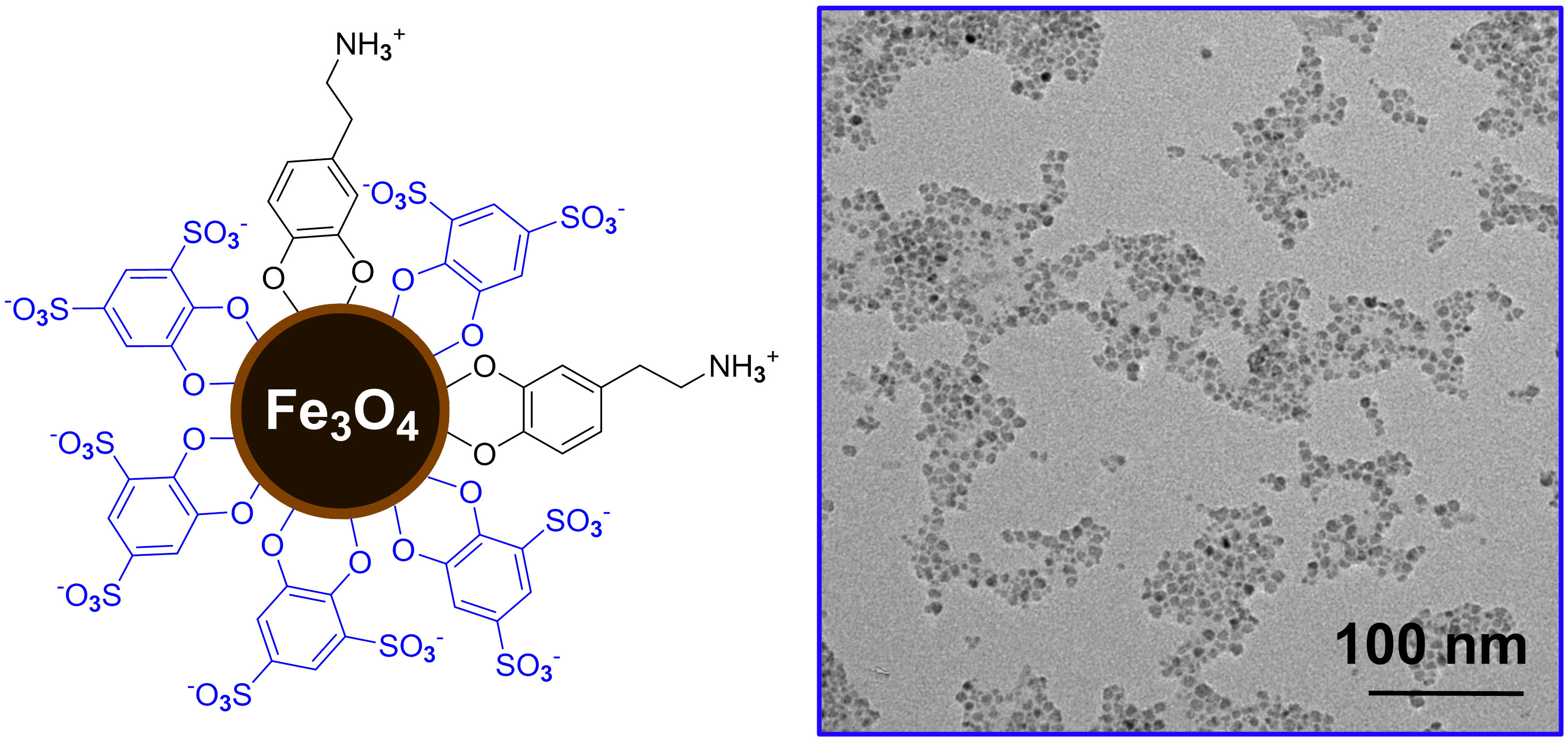
Aqueous stable superparamagnetic iron oxide nanoparticles (IONPs) are a key component in many current technologies, such as MRI contrast agents, magnetic separations, drug targeting, and hybrid inorganic-organic nanomaterials. As IONP properties depend on particle size, stability, and ligand shell identity, these parameters must be strictly controlled for their successful use as functional materials. Ultrasmall (<15 nm) nanoparticles are required to facilitate downstream nanomaterials applications (that require templation on a small size regime) or to maintain desired superparamagnetic properties. As MRI contrast agents, smaller IONPs possess a longer half-life in the bloodstream. Particles with a diameter <10 nm can penetrate the endothelium and show increased bioavailabilty. Synthesis of ultrasmall IONPs that are stable in aqueous solution is critical for bio-medical applications.
This project works to develop a systematic understanding of the factors controlling surface structure, aqueous solubility, stability and degradation pathways of IONPs, which will have an immediate impact on biomedical imaging. IONPs are composed of an inorganic iron oxide (typically Fe3O4) core protected by surface capping ligands and, due to their unique combination of magnetic properties, low biological toxicity and low cost, have been recognized as ideal components for advanced technologies including magnetic resonance imaging (MRI) contrast agents, magnetic separations, and drug targeting. Unfortunately, the inability to readily and reliably generate IONPs with different capping ligands using conventional, solution-based techniques has severely hindered the development of IONP technologies and has largely prevented the development of a systematic understanding of how the key properties of IONPs, particularly surface structure and stability, can be modified and optimized by the choice and structure of capping ligands.
We also tune the magnetic properties of iron oxide nanoparticles by adding dopants such as Co and Gd during synthesis. The method we use enables the incorporation or 2+ or 3+ metal ions, replacing some of the iron. Our relatively low temperature (~75 C) synthesis also uses safer salt precursors and non-toxic ligands. In the Blum group, we have developed a general, low temperature, and simple protocol for exchanging the oleic acid ligands used in nanoparticle synthesis to generate monodisperse particles with catechol-based ligands containing a variety of functionalities. We explore new surface chemistries, and seek to understand the principles which govern surface interactions between iron oxide and organic ligands to control nanoparticle properties.
Tuning the optical properties of plasmonic nanoparticles

There is a great deal of interest in the controlled synthesis of noble metal nanoparticles that can be largely attributed to their versatile applications in various fields including electronics, catalysis, optics, biological labeling and surface-enhanced Raman spectroscopy (SERS). Nobel metal nanoparticles can support localized surface plasmon resonances (LSPRs) - coherent oscillations of conduction band electrons in response to specific wavelengths of light - which can result in an extraordinary enhancement of local electromagnetic fields in the junction between two adjacent metal particles. These junctions or “hot spots,” have a focusing effect on electromagnetic field intensity. Although silver nanoparticles are of great interest due to their properties as SERS enhancers and due to their plasmon resonance in the blue wavelengths, not much is known about reliably controlling their assembly into extended structures to tune their optical properties. The controlled organization of plasmonic nanoparticles into highly anisotropic 1D arrays and networks offers a platform to attune the flux of surface plasmons. Extended planar nanoparticle assemblies are capable of subwavelength optical guiding, which can result in the miniaturization of integrated optical, photonic and biosensor devices.
This project is based on gold, silver, and gold-silver alloy nanoparticles. When working with silver, we use a green and facile aqueous synthesis of silver nanoparticles by chemical reduction and their stabilization by four short molecules: glycine, cysteine, cysteamine, and dithiothreitol (DTT). We can generate extensive 1D self-assembled superstructures of metallic silver nanoparticles by controlling the degree of nanoparticle surface passivation and polarization by either of the three ditopic ligands: cysteine, cysteamine and dithiothrietol. UV-Vis reveals the characteristic surface plasmon band of silver colloids (SP) centered at 390 nm as well as a low-energy longitudinal plasmon band (LP) that is suggested to arise from uniaxial coupling of the isotropic surface plasmons. The plasmon coupling is reinforced by the hydrogen-bonding offered by the end moieties of the ligands chosen. The spontaneous formation of the chains is due to the alignment of dipoles within short inter-dipole distances to minimize the enthalpy cost as well as the disorder provided by the branched domains.
We also work with gold-silver alloy particles grown through a seeded growth method. These particles can have plasmon resonance tuned between ~390 nm (pure silver) and ~530 nm (pure gold). We hope to combine the advantages of gold - stability, well-establshed surface chemistry, non-toxicity with the advantages of silver - stronger plasmon resonance and cheaper. In addition, both silver and gold nanoparticles have catalytic activity that might be tunable or enhanced with alloy nanoparticles.