Confronting Neurodegeneration: Insights into Parkin Function as a Protective Enzyme in Parkinson’s Disease
By: Jonathan Krett
(Supervisor: Dr. Edward Fon)

Parkinson’s Disease (PD) is the second-most common neurodegenerative condition behind Alzheimer’s, affecting one percent of the population over the age of 65. This statistic rises with advanced age, affecting five percent of individuals 85 and older1,2. Patients with PD are afflicted by a slew of motor and non-motor symptoms which include tremor at rest, slowness of movement, and cognitive impairment3. PD is characterized by the progressive death of dopamine-producing neurons in the substantia nigra pars compacta (SNc) of the midbrain and the appearance of protein aggregates called Lewy Bodies within the cells. While the loss of dopamine neurons with age occurs in all individuals, PD patients are more vulnerable to premature or excessive cell death in the SNc. This exaggerated neuronal degeneration correlates with their clinical presentation4. A combination of genetic and environmental factors determines whether PD symptoms will manifest in a given individual prior to the end of life. Therefore, Parkinson’s is a disease of aging featuring a strong contribution of factors other than advanced age, which together act to circumscribe dopamine neuron loss3. Presently, there is no treatment for PD that can slow or reverse the course of neurodegeneration. Clinicians instead do their best to target the most troublesome symptoms with dopamine-replacement therapy (e.g. L-DOPA administration), or with surgical interventions such as deep brain stimulation (when symptoms don’t improve with medication). The goal of much current research then is to (eventually) develop treatment options capable of modifying the actual course of dopamine neuron degeneration for the benefit of PD patients.
Contemporary research investigating the neurobiology of PD progression implicates the maintenance of the mitochondrial network as a crucial determinant of cell survival in the SNc5. Toxins of the respiratory chain within mitochondria such as MPTP, rotenone, and paraquat, have been associated with elevated dopaminergic cell death and oxidative stress. In addition, oxidative stress concomitant with mitochondrial dysfunction may interact with abnormalities in protein waste management that are an integral part of PD neuropathology (i.e. Lewy Body formation)6. Animal models beginning in the early 2000’s demonstrated that the autosomal recessive PD genes PINK1 (encoding a mitochondrial kinase) and parkin (encoding an E3 ubiquitin ligase) operate together in a pathway that maintains the health and normal function of mitochondria, with parkin likely acting downstream of PINK17,8. Subsequent studies in mammalian cells demonstrated that parkin mediates the engulfment of impaired mitochondria by the autophagy pathway. This is one avenue in which the cell can quarantine and destroy hazardous material from within to protect itself from further damage. For impaired mitochondria, this process is specifically termed mitophagy, and the action of parkin here is dependent on PINK19. Parkin responds to mitochondrial damage following PINK1 accumulation at the outer mitochondrial membrane when these organelles have lost their membrane potential10. In recent years, it has come to light that parkin manages a portfolio of mechanisms that act to maintain mitochondrial health, termed mitochondrial quality control5,11. Through these mechanisms, parkin may play a major role in safeguarding neurons, raising the exciting possibility that parkin activity could be fine tuned as a therapeutic strategy in PD.
Wielding the recently discovered full-length crystal structure of parkin12 as a framework, the goal of my project was to compare the function of mutant forms of the enzyme to the wild type form. Mutations were engineered to alter amino acids in parkin domains that we hypothesized would impact its ability to carry out crucial enzymatic functions. After expressing parkin in mammalian cancer cell lines, I examined the performance of mutant and wild type parkin at two major endpoints: (a) in mitochondrial translocation (recruitment) and (b) eventual clearance of the organelles (mitophagy), in response to a chemical trigger that simulates the effects of severe damage to mitochondria.
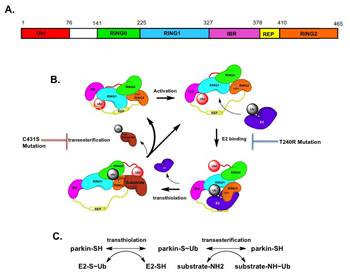
The ubiquitin ligase cycle of parkin proceeds like a game of “hot-potato,” where an E2 conjugating enzyme will first pass ubiquitin (the “potato”) to the active site of parkin (located at cysteine-431; see figure 1B). Following this, parkin will pass ubiquitin to a substrate protein13. Ubiquitin can then act to target a protein for degradation or promote mitophagy, amongst other pathways. There is evidence that in biochemical reactions carried out in a test tube (in vitro), the first step of this “hot potato” cycle, called transthiolation, is rate-limiting. On the other hand, the subsequent step (transesterification) likely occurs at a greater rate12. In my experiments in living cells, I found that PD-causing mutations which interfere with the initial step of E2-parkin binding (at threonine-240/T240), greatly impair parkin recruitment and mitophagy, measures of its ability to address mitochondrial damage in our model. Close-by mutations at this site in parkin at threonine-242 and aspartate-243, which abolish parkin activity in vitro12, were notably capable of responding to mitochondrial damage with only a brief delay compared to wild type parkin. By contrast, single mutations that selectively impair the transesterification step of catalysis, for instance a mutation at histidine residue 433, do not detectably slow down parkin recruitment in cells.
Overall, these findings advance evidence that the transthiolation step in parkin catalysis is rate-limiting for the response of this enzyme to mitochondrial distress in cells. The results here suggest that if we are to upregulate parkin activity in PD brains as a neuroprotective strategy, for example with small molecule drugs, the transthiolation step would serve as a robust focus for manipulation. Further elucidation of parkin’s role in mitochondrial quality control mechanisms may bring us closer to finding a tool to slow PD progression by helping vulnerable neurons survive. With our continued efforts, we aim to give these neurons the boost they need to halt neurodegeneration in its tracks.
Figure 1: Parkin Structure and Catalysis. Parkin is a member of a family of RING-In Between-RING (RBR) E3 ubiquitin ligases. It possesses a ubiquitin-like domain (Ubl), followed by RING0, RING1, and an In-Between-RING (IBR) linker domain. The IBR domain extends into the Repressor Element of Parkin (REP), which acts to suppress parkin activity by binding to RING1. The C-terminal RING2 domain houses the active site cysteine-431 (C431)12. (A) Depicts the domains of parkin as modules. (B) Demonstrates the catalytic cycle of parkin and a ‘bean’ interpretation of its structure, as identified by Trempe and colleagues in 201312. Threonine-240 (T240) in RING1, a site mutated in some familial forms of Parkinson’s Disease forms a major interface for binding of E2 conjugating enzymes and is critical for binding of these enzymes to parkin. The final step, transesterification, is impaired by mutation of the active site C431. (C) Parkin catalysis divided into two main steps: transthiolation and transesterification. Transthiolation, which involves binding of an E2 enzyme and removal of the REP at RING1 is rate-limiting for the recruitment of parkin to mitochondria when they are damaged. Images are adapted from the work of Dr. Jean-François Trempe, McGill University.
References
1. Van Den Eeden, S. K. Incidence of Parkinson's Disease: Variation by Age, Gender, and Race/Ethnicity. American Journal of Epidemiology 157, 1015–1022 (2003).
2. de Lau, L. M. & Breteler, M. Epidemiology of Parkinson's disease. The Lancet Neurology 5, 525–535 (2006).
3. Shulman, J. M., De Jager, P. L. & Feany, M. B. Parkinson's Disease: Genetics and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 6, 193–222 (2011).
4. Kansara, S., Trivedi, A., Chen, S., Jankovic, J. & Le, W. Early diagnosis and therapy of Parkinson's disease: can disease progression be curbed? J Neural Transm 120, 197–210 (2013).
5. Narendra, D., Walker, J. E. & Youle, R. Mitochondrial quality control mediated by PINK1 and Parkin: links to Parkinsonism. (2012). Cold Spring Harbor Perspectives in Biology.
6. Exner, N., Lutz, A. K., Haass, C. & Winklhofer, K. F. Mitochondrial dysfunction in Parkinson's disease: molecular mechanisms and pathophysiological consequences. EMBO J 31, 3038–3062 (2012).
7. Greene, J. C. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proceedings of the National Academy of Sciences 100, 4078–4083 (2003).
8. Park, J. et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature (2006).
9. Narendra, D., Tanaka, A., Suen, D.-F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. The Journal of Cell Biology 183, 795–803 (2008).
10. Narendra, D. P. et al. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol 8, e1000298 (2010).
11. Mclelland, G.-L., Soubannier, V., Chen, C. X., Mcbride, H. M. & Fon, E. A. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J n/a–n/a (2014).doi:10.1002/embj.201385902
12. Trempe, J. F. et al. Structure of Parkin Reveals Mechanisms for Ubiquitin Ligase Activation. Science 340, 1451–1455 (2013).
13. Wenzel, D. M., Lissounov, A., Brzovic, P. S. & Klevit, R. E. UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids. Nature 474, 105–108 (2011).