TMC9 as a novel mechanosensitive ion channel
By: Lou Beaulieu-Laroche
(Supervisor: Dr. Reza Sharif-Naeini)

Mechanical forces play numerous roles in physiology. When an object contacts our skin, it exerts a force that is encoded as touch or pain depending on its intensity. Likewise, sound waves physically move cell processes in the inner ear, enabling us to hear. Forces intrinsic to the human body are also important. For instance, blood pressure is tightly regulated by mechano-sensitive feedback processes. Mechanotransduction, the conversion of mechanical stimuli into electrochemical signals, is ubiquitous, but the molecular players are vastly unknown. Because mechanotransduction can be exquisitely fast, it is believed to be mediated by ion channels rather than second messengers (Corey and Hudspeth, 1979). Mechanosensitive ion channels (MSCs) are thought to rapidly transform mechanical deformations of the plasma membrane into electric currents. However, very few MSCs have been identified, precluding a precise investigation of the physiology and pathophysiology of the different mechanotransduction processes.
Through a previous screen of candidate genes, the laboratory of Dr. Sharif-Naeini isolated TMC9 as a putative MSC. TMC9 is well conserved amongst mammals and is present in several tissues, but its function is unknown. For my honours project, I joined the laboratory to investigate the role of TMC9 in mechanotransduction.
The first part of my project aimed at determining whether TMC9 formed a MSC in heterologous systems. I performed electrophysiological experiments with Chinese hamster ovary (CHO) cells transfected with a control plasmid or a plasmid expressing TMC9. Mechanosensitive currents were elicited by applying pulses of negative pressure through the recording electrode while recording in the cell-attached patch clamp configuration. Overexpressing TMC9 in CHO cells substantially increased the mechanosensitive currents. To determine whether this was due to TMC9 forming a channel or simply modulating the activity of endogenous channels, I broke down the patch recordings into two categories: active and silent patches. Active patches had at least one active MSC, while silent patches had none. There was a higher percentage of active patches and the currents in the active patches were significantly enhanced in cells overexpressing TMC9, suggesting a higher channel density at the plasma membrane.
I then measured the activation threshold, single-channel conductance, reversal potential and calcium permeability of the mechanosensitive ion channels in control and TMC9-transfected cells. Similar results for both groups suggest that expressing TMC9 enhanced the number of mechanosensitive ion channels that are endogenously expressed by CHO cells. To confirm that TMC9 indeed forms an ion conduction channel, our laboratory purified it and reconstituted it in artificial lipid bilayers. Recordings from those bilayers demonstrated that TMC9 forms an ion conducting pore, and is therefore a novel ion channel.
This first set of experiments demonstrated that TMC9 can form a mechanosensitive channel, but its physiological role remained unknown. Thus, I next investigated the contribution of TMC9 to different mechanotransduction processes. Somatosensation, which comprises bodily sensations such as touch, pain and proprioception, relies heavily on mechanotransduction. The neurons involved in somatosensation are located in dorsal root ganglia (DRG), which sit adjacent to the spinal cord. DRG neurons send one axon to the periphery to sense the external environment and another one to the central nervous system to convey information.
Piezo2, one of the few MSCs known to date, is expressed by a subset of DRG neurons and has been found to be the main transducer of light-touch (Ranade et al., 2014). However, the channels mediating pain mechanotransduction remain elusive. Previous recordings from painsensing DRG neurons (nociceptors) unveiled that they possess high threshold, low conductance MSCs, but their genetic identity is unknown (Cho et al., 2002; Drew et al., 2002; Sharif-Naeini, 2015). Interestingly, TMC9 overexpression in CHO cells induced the expression of MSCs with similar activation threshold and conductance. Moreover, TMC9 is endogenously expressed in DRG neurons. We thus asked whether TMC9 could be involved in the transduction of painful mechanical stimuli. I recorded mechanosensitive currents from dissociated nociceptors that were treated with mock or TMC9-targetting siRNA. Knocking down TMC9 significantly reduced the mechanosensitive currents. Moreover, the percentage of active patches and the currents in the active patches were smaller. Overall, TMC9 appears to be an important player in nociceptive mechanotransduction.
Because those experiments were performed in vitro, further in vivo experiments are needed to assert that TMC9 is involved in our ability to sense mechanical pain. Nonetheless, identifying the MSCs responsible for pain transduction in vivo would have important implications for pathological pain conditions. For instance, the sensitization of MSCs has been implicated in osteoarthritis, a degenerative joint disease characterized by painful movements (McDougall, 2006; Sharif-Naeini, 2015). In addition to pain-inducing stimuli being more painful in osteoarthritis, stimuli that normally do not elicit painful responses are now painful, a condition named allodynia. This allodynia can be explained by the sensitized MSCs getting activated by innocuous movements. Knowing the identity of the channels would greatly help investigate the contribution of MSCs to osteoarthritis and other diseases with similar underlying pathophysiology. Moreover, it would provide an interesting therapeutic target to treat such pathologies.
My honours project in Dr. Sharif-Naeini has been very exciting and productive. Before engaging in graduate studies at the Massachusetts Institute of Technology, I am conducting a summer research project funded by an NSERC award in Dr. Sharif-Naeini’s laboratory. In addition to analyzing the role of TMC9 in more physiological conditions to cogently show that the channel is important in vivo, I plan to decipher the precise mechanism by which the channel transduces mechanical forces into electric currents using site-directed mutagenesis.
Click image for larger version
Figure legend
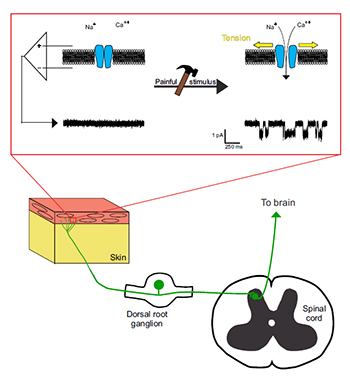
Model for the role of TMC9 in the transduction of painful mechanical stimuli. In the periphery, a nociceptor innervates the skin with free nerve endings. When a painful mechanical stimulus is sensed by the nerve terminals, the tension in the membrane increases, forcing TMC9 to open and flux cations (red box). This flow of positive charges can be measured electrophysiologically, with typical square-shaped single channel openings shown on the right. When the stimulus is strong enough, an action potential is generated and reaches the cell body of the nociceptor, which sits in a dorsal root ganglion. The electrical signal is then propagated to the dorsal horn of the spinal cord, where it is processed and sent to the brain.
References
Cho H, Shin J, Shin CY, Lee SY, Oh U (2002) Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 22:1238-1247.
Corey DP, Hudspeth AJ (1979) Response latency of vertebrate hair cells. Biophysical journal 26:499-506.
Drew LJ, Wood JN, Cesare P (2002) Distinct mechanosensitive properties of capsaicin-sensitive and - insensitive sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 22:Rc228.
McDougall JJ (2006) Arthritis and pain. Neurogenic origin of joint pain. Arthritis research & therapy 8:220.
Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A (2014) Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516:121-125.
Sharif-Naeini R (2015) Contribution of mechanosensitive ion channels to somatosensation. Progress in molecular biology and translational science 131:53-71.