Our capstone projects have a focus on medical technologies and devices, as well as health and multidisciplinary projects. Projects come from academics and companies, thereby giving students the opportunity to work on and provide solutions to relevant issues and questions.
Project List
1—Clemex Microscope Enclosure
Client
Contact
matthieug [at] clemex.com (Matthieu Guihard)
Project
Clemex is presently building its own microscope dedicated to specific needs, the objective being to reduce the overall cost of such system for a particular industry.
As seen in the image, the system involves a X/Y stage, a light path (objective, lens and tube, camera, ring light) and a motorized Z-Axis, all assembled on a common plate.
The proof of concept has been done and works. Only the motorized Z-axis will be changed during the summer rendering the system more compact.
Next step of the project will be to design and build an appealing enclosure that merges functionalities such as hardware settings, stage mobility, sample accessibility, visual appearance and other requirements as described in a future specifications document.

2—Development of a Test Load for Whole Body Plethysmography
Client
Contact
taylor.wilson [at] scireq.com (Taylor Wilson)
Introduction
SCIREQ Inc. is a recognized world leader in the respiratory research community as a producer of innovative tools that help scientists acquire novel insights into the lungs. The use of rodents and other small animals in respiratory research has been vital in leading to scientific discovery and development benefitting humankind. Whole body plethysmography (WBP) is a standard method for studying pulmonary function in conscious, spontaneously breathing laboratory subjects. The barometric plethysmography technique measures flow and pressure changes that occur while the subject is breathing, before and after exposure to a pharmaceutical or other challenges. WBP is the least invasive method of studying pulmonary function and consists of placing the subject in a chamber, where they can move freely and explore while a pressure transducer measures the flow and pressure changes caused by their breathing. It is often used for longitudinal studies where the subjects are studied for multiple hours on successive experiment days.

Figure 1: SCIREQ mouse WBP chamber
Why a Test Load?
In plethysmography, it is difficult to troubleshoot some issues because the signals generated by the subjects can be noisy due to a number of factors such as the lab environment, movement of the subject etc. A test load is used to model the the expected usage of equipment, by simulating the signals the equipment is designed to measure. For the case of WBP, the test load would need to simulate a small animal’s breathing patterns. Currently, we are using a 1 mL syringe to simulate a signal for our WBP by oscillating the plunger rapidly, which is not very accurate. It is difficult to accurately test the system when the signal itself is not consistent. Having a characterized test load will help with in-house testing, calibration and trouble shooting at customer sites.
Capstone Project
The goal of the CAPSTONE project is to develop a test load for use with WBP systems. The test load should be able to generate flows using an actuator at various frequencies. The design must meet a specific set of criteria in the areas of size, flow generation, system integration, manufacturability, cost, and ease of use. Your design will be tested by SCIREQ engineers and has the potential for becoming a standard product that is shipped with every WBP sale.
You will get to work with our team of engineers in a collaborative Agile work environment and be able to leverage our manufacturers’ capabilities to make your design a reality. As your mentors, we are available to you for questions, discussion, and regular project meetings. We are looking for an innovative, motivated team who want to make a global impact with this practical research application. Working with SCIREQ, you will gain valuable hands-on experience, joining a diverse and inclusive team whose philosophy is rooted in courtesy, honesty, integrity, and fairness.
3—Development of Rat "Soft Restraint" for Use in Inhilation/Exposure Tower
Client
Contact
taylor.wilson [at] scireq.com (B)ben [at] scireq.com (en Urovitch)
Introduction
SCIREQ Inc. is a recognized world leader in the respiratory research community as a producer of innovative tools that help scientists acquire novel insights into the lungs. The vital use of rodents and other small animals in respiratory research has led to scientific discovery and development benefitting humankind. One typical application is inhalation/exposure, where subjects are exposed to a controlled atmosphere, exploring the effects of external compounds (e.g., toxins, pharmaceuticals). Controlled inhalation/exposure is used to research asthma, pulmonary disorders, infectious disease, air pollutants, tobacco, cannabis, vaping, pharmaceutical development, among others. One method of controlled delivery is via a nose-only inhalation setup. Subjects are restrained, limiting exposure to their snout and allowing for more accurate results due to limited body exposure, more control, and lesser quantities of pharmaceuticals or toxins being used.
Restraints in Mice
An industry standard for exposure studies involves nose-only inhalation restraining devices which are typically rigid, fully enclosed, and include a plunger that forces the subject forward. This form of restriction may result in the subject producing irregular body heat or abnormal breathing. In extreme cases, the subject may attempt to retreat within the restraint, causing itself harm.

Nose-only inhalation restraining devices
SCIREQ has developed SoftRestraints for mice to minimize some of the negative effects of typical restraints. The open mesh has proven to be gentler yet secure, imposing little-to-no compression to the torso and therefore does not impede chest movements. Subjects body is also exposed to the environment allowing for better heat dissipation.

SoftRestraint for mice
Capstone Project
The goal of the CAPSTONE project is to develop a soft restraint for rat use. Rats have unique challenges for restraining; their size, weight, and temperament being some. The restraint may follow SCIREQ’s pre-existing technology or could be an entirely new design. The approach and strategy is up to you. The design must meet a specific set of criteria in the areas of subject sizes, system integration, ability to sterilize, manufacturability, and ease of use. Consequently, your design will be tested by the technicians that work with live subjects and the researchers who use SCIREQ’s equipment.
You will get to work with our team of engineers in a collaborative Agile work environment and be able to leverage our manufacturers’ capabilities to make your design a reality. As your mentors, we are available to you for questions, discussion, and regular project meetings. We are looking for an innovative, motivated team who want to make a global impact with this practical research application. Working with SCIREQ, you will gain valuable hands-on experience, joining a diverse and inclusive team whose philosophy is rooted in courtesy, honesty, integrity, and fairness.
4—Design and Optimization of Thought Technology eVu-TPS Physiological Sensor
Client
Contact
hal [at] thoughttechnology.com (Dr. Hal Myers)
Project
eVu-TPS was initially designed in 2007 and was one of the first Bluetooth-enabled finger-worn physiological monitoring devices. The unit monitors heart rate/HRV, finger temperature, skin conductance, and respiration from one fingertip and uses apps on Android, iPhones and PC’s to acquire and provide Biofeedback. It has been designed, along with all our equipment, to comply with medical regulatory requirements in many countries.
We are seeing an uptake by clinicians in the use of the devices to remotely train their patients, mostly because of Covid-19, because of the relatively low cost and ease of use compared to our general line of products.
Currently, our manufacturing cost with all packaging and isolated charger costs is too high compared to desired market retail price. Our engineering team believes this cost is achievable with some of the following changes:
- Change the design from an architecture incorporating front end signal monitoring and a separate analog to digital converter and microprocessor to using the capabilities of some BLE Bluetooth chips to perform these functions, thereby eliminating a significant amount of circuitry.
- Since the original design incorporated a standard Bluetooth module, it required a battery capable of significant power. The new design will use BLE, so the size and cost of the battery can be reduced.
- Change the PPG (photoplethysmograph) monitoring from discrete Infrared LED/photocell components to an inexpensive all-in-one module that monitors not only PPG, but also oximetry.
- Consider incorporating EKG monitoring using a finger on the opposite hand touched to a conductive part of the case.
- Redesign the printed circuit board layout and finger plate electrodes to be easily manufacturable in quantity.
- Redesign the case to be thinner and less obtrusive.
- Redesign the charging technique. The goal is to charge directly from a 5v USB-type source to a case that would isolate the fingers from the electrodes.
Preferable Qualifications of ECE Students
- Electronic design experience, ideally for low-level biologic signals
- Firmware experience
- Some app development skills - although the app is already developed, we may need to add the extra EKG signal.
Preferable Qualifications of MECH Students
Industrial design to make the case smaller and easier to manufacture
Resources
- Parts for prototypes
- Guidance by an experienced team of electrical, mechanical, and firmware engineers. The exciting opportunities for those working on this project includes the opportunity to design a product for real-world applications, thus preparing them to move easily into companies requiring these skills.
Non-Disclosure Agreement Requirement
Since this project is a currently sold product, we require a non-disclosure agreement from students and faculty working on the project, and the requirement that if students want to present the project to others, that circuitry be shown only in block diagram form without schematics or specific components or firmware.
Links to Some of Thought Technology’s History and Products
eVu-TPS: Triple-Physiology Sensor
Reward and Signal view of eVu-Senz App on Android
How to work remotely using ZOOM
MyOnyx 4-Channel Encoder System: 1 minute overview
Dr. Hal Myers presenting about Biofeedback and Neurofeedback at a McGill EE lecture
5—Design and Implementation of State-of-the-art Medical Device MEMS Sensor Interface
Client
Contact
nizar.kezzo [at] nxtsens.com (Nizar Kezzo)
Project
An emerging field of microscopic devices that combine both mechanical and electrical components is seen across a wide range of industries including the biomedical field. These devices are known as Micro Electro-Mechanical System or MEMS. MEMS are able to sense, control and actuate on the micro scale and generate effects on the macro scale [1]. The highly miniaturized and integrated MEMS devices are used to add ‘eyes and ears’ to medical equipment. The ability to actively monitor biometric patient data in real-time can provide great benefit to physicians, first responders, and medical professionals everywhere in their mission to improve patient outcomes.
Our flagship product, the MY01 device, is one such example. MY01 is an FDA approved biomedical pressure monitor, which functions by inserting an active pressure sensor into the patient’s muscle. Continuous pressure readings serve as an aide to diagnosis of Acute Compartment Syndrome (ACS) [2], which is dangerous and difficult-to-detect condition prevalent in patients suffering from high-energy trauma such as bone fractures.
The MY01 device uses a MEMS sensor to measure pressure in the patient’s muscle. MY01 Inc. is seeking to further improve the accuracy and reliability of the MEMS element to deliver next generation ACS diagnostic tools to the hands of physicians. The scope of the project includes designing a MEMS sensor interface development board alongside a user application. The development board shall explore different MEMS sensor and interface architectures. This will involve an embedded system design.
We are seeking a team to work with us in developing new and improved ways of interfacing technology with the human body. This project aims to further unlock the potential of modern sensors in the biomedical field by offering enhanced visibility in difficult-to-access areas of our anatomy.
[1] "An Introduction to MEMS (Micro-electromechanical Systems)," PRIME Faraday Partnership, 2002.
[2] C. P. M. Osborn and A. Schmidt, "Management of Acute Compartment Syndrome," JAAOS - Journal of the American Academy of Orthopedic Surgeos, vol. 28, no. 3, pp. e118-e114, 2020.
6—Conceptual Design of Cutting-Edge Insertion Methods for Modern Biomedical Sensors
Client
Contact
christopher.agellon [at] nxtsens.com (Christopher Agellon)
Project
Advancements in modern sensing technology creates the need for new and innovative methods for in-vivo implantation of biomedical sensing elements. The ability to actively monitor biometric patient data in real-time can provide great benefit to physicians, first responders, and medical professionals everywhere in their mission to improve patient outcomes.
MY01 Inc. is seeking to design and prototype safe and effective tools to “introduce” or “insert” our cutting-edge sensors into patients in need of enhanced monitoring capabilities. The envisioned system should be portable, light-weight, intuitive to use, and can be administered with a high degree of reliability.
Our flagship product, the MY01 device, is one such example. MY01 is an FDA approved biomedical pressure monitor, which functions by inserting an active pressure sensor into the patient’s muscle. Continuous pressure readings serve as an aide to diagnosis of Acute Compartment Syndrome (ACS) [1], which is dangerous and difficult-to-detect condition prevalent in patients suffering high-energy trauma such as bone fractures.
We are seeking a team to work with us in developing new and improved ways of interfacing technology with the human body. This project aims to further unlock the potential of modern sensors in the biomedical field by offering enhanced visibility in difficult-to-access areas of our anatomy.
To learn more, visit our website.
[1] C. P. M. Osborn and A. H. Schmidt, "Management of Acute Compartment Syndrome," JAAOS - Journal of the American Academy of Orthopaedic Surgeons, vol. 28, no. 3, pp. e108-e114, 2020, doi: 10.5435/jaaos-d-19-00270.
7—Design of an Ophthalmic Imaging Device with Data Processing Algorithms
Client
Contact
oliver.wumartinez [at] mail.mcgill.ca (Oliver Wu Martinez), jeremy.zwaig [at] mail.mcgill.ca (Jeremy Zwaig), angela.wong2 [at] mail.mcgill.ca (Angela Wong), athithan.ambikkumar [at] mail.mcgill.ca (Athy Ambikkumar), leonard.levin [at] mcgill.ca (Dr. Leonard Levin)
The Motivation
Currently in North America, there is a lack of ophthalmologists. We are creating a remote and asynchronous eye exam to assist ophthalmologists and help patients. Patients will access our imaging device at their local medical centers. The captured data will then be sent to an ophthalmologist who can diagnose the patient equivalently to an in-person slit lamp exam.
Objectives
Hardware component (2 MECH students): Students will be tasked to build a state-of-the-art imaging device utilizing pre-existing imaging technology that has not been exploited in the field of ophthalmology. Students will use their experience and creativity to design, build and optimize an optical system with this new technology for front of the eye diagnosis.
Software component (2 ECE students): Develop a data processing method for the raw data collected by the novel imaging device. Downstream goals for the software will be to manipulate and transform imaging data to allow for a dynamic eye exam with a user interface equivalent to the slit lamp exam.
Requirements: Looking for students with an interest in domain-specific data compression, use of GPU, field logic array, mathematics, Python or C, and/or miniaturization (previous experience in any of these fields is an asset).
8—Design and Integration of an Automatic Sash Positioning System
Client
Contact
rrivera [at] bedco.ca (Robert Rivera)
Project
Bedcolab is a manufacturer of laboratory casework systems and fume hoods for research centers. We service universities, the pharma and biotech industries as well as government and other industrial labs.
We are looking to update our Vanguard line of fume hoods. Our objective is twofold. Technically, we wish to design and integrate a more cost-effective sash positioning and closing system that would involve motion detection and possibly electro-magnetic applications. From a design perspective, our objective is to give our hood facing a more technical appearance. Because the sash positioning system will be integrated in the sash, and will impact the esthetics of the hood facia, we feel that both objectives will need to be addressed simultaneously.
The project will involve two teams: Mechanical Engineering and Electrical and Computer Engineering. Both teams should work in close collaboration and coordination.
Deliverables
- To design a sash positioning mechanism and integrate it into existing fume hood (MECH);
- To modify existing design of the fume hood, as needed, to accommodate the new positioning system without altering the performance of the fume hood (MECH);
- To select and integrate sensors needed for the position control (MECH, ECE);
- To develop control algorithms for sash positioning with motion detection feedback (ECE);
- To design and integrate electrical/electronic control system (ECE).

Current state of Vanguard fume hood
9—Design and Validation of a Minimally Invasive Hallux Valgus Correction System
Client
Contact
melinda.a [at] pegamedical.com (Melinda Ayvazian, Eng.)
Background
Under the supervision of Pega Medical, the engineering team will be challenged to develop and optimize the design of implants and instruments necessary for the minimally invasive treatment of Hallux Valgus, based on the Bosch percutaneous technique, and device idea presented by Dr. Gdalevitch, MD, FRCS(C).
Objectives
The objectives of the project are to work within ISO13485 requirements to build the DHF/DMR of the product, complete the design of a minimally invasive implant, with possible IP protection, complete the design of a functional external fixator system and all annex instrumentation, and validate the design in the operating theater with the collaborating surgeon.
Description of Design Component
PHASE 1: Design Inputs and Schedule
- Development of design inputs for the implant and instruments
- Establishing a project schedule
PHASE 2: Preliminary Design of Implants
- Development of ideas for the implant (brainstorming)
- Preliminary evaluation of risks/ potential harms
- Design Review for selection of preliminary concept and refinement of selected concept
- Patent research and preliminary drafting
PHASE 3: Preliminary Design of Instruments
- Development of ideas for the instrumentation (brainstorming)
- Drafting of preliminary Surgical Technique
- Design Review for selection of preliminary concept and refinement of selected concept
PHASE 4: Preliminary Prototype Manufacturing and Verifications
- Design and Development of 3D functional models
- Establishing the validation plan for verifications (FEA, Calculations, etc) and bench testing validations (design and quoting of simplified prototypes, design of testing jigs, development of testing protocols)
- Rapid prototyping of parts (if applicable)
- Testing of preliminary implant models and instrument models
- Clinical validation of system with collaborating surgeon
PHASE 5: Final Concept of the Implant
- Optimization of final concept of the implant
- Preparation of engineering drawings
- Update to risk management
PHASE 6: Final Concept of the Instruments
- Optimization of final concept of the implant
- Preparation of engineering drawings
- Update to risk management
Economic and Societal Impacts
The current market for Hallux Valgus correction proposes over a hundred different techniques, but no clear consensus on the best approach to treatment. The majority of techniques are open techniques with either plate or screw devices being used for fixation of the bone’s segments after correction of the IM and HV angles. The objective of the new medical device would be to offer a unique percutaneous device that will correct the deformity by application of translation and rotation around the CORA, maintain the ROM of the first toe, reduce affect on surrounding soft tissues, reduce pain of the patient, and reduce the risk of reoccurrence of the deformity.
Requested or To Be Developed Skills of the Student Team
Creativity, problem-solving skills, mechanical CAD design (Solidworks), organizational approach, structural analysis, teamwork, communication skills
10—OpSens Guidewire Shaping Tool
Client
Contact
maxime.pdeland [at] opsens.com (Maxime Picard-Deland), Medical Technology Specialist
Project
OpSens Inc. is a manufacturer of interventional guidewires instrumented with fiber-optic sensors. One application of such wire is to support the delivery of heart valve prosthesis in a patient by a transcatheter approach, i.e. by guiding the prosthesis in the blood vessels. This minimally invasive procedure allows to replace a diseased heart valve through a small incision in the patient skin, instead of an open-chest surgery. The pressure sensor at the tip of OpSens guidewires allows to measure the pressure drop induced by the diseased valve, and to compare it with that of the new prosthesis. This pressure gradient and other pressure-based clinical metrics are important information to guide the physician clinical decisions.
OpSens has already developed a straight guidewire for the replacement of aortic valves. The company wants to develop a new guidewire with a curved shape that would allow to guide valve prosthesis through more complicated access, such as the transseptal access for mitral valve replacement. Ideally, the straight guidewire would be modified by a tool at the end of the fabrication steps to give it the desired curved shape, thus minimizing modifications to assembly lines. This tool could also be provided directly to the hospitals, allowing the physician to shape the guidewire with a curve specific to the patient anatomy.
The main objective of the project is to design a tool that allows shaping a curve into a 0.035" OD guidewire by inducing a controlled plastic deformation to the guidewire, without damaging the PTFE coating. Secondary objectives are: 1) to model the shaping parameters with the guidewire mechanical properties, allowing to shape different guidewire products into different curves, and 2) to provide a tooling concept that can be used in a sterile environment by the end-user (the physician).
11—Development of Anthropomorphic and Tissue-Mimicking Dynamic Arterial Phantoms
Client
rosaire.mongrain [at] mcgill.ca (Prof. Rosaire Mongrain)
Project
For surgical training, virtual surgical planning and numerical model validation, reproducible synthetic arterials mockups (phantoms) are needed. These models need to replicate the mechanical properties of native tissue (hyperelastic, anisotropic, heterogeneous). The large deformation, the layered structure and pathological degradation of the vessel need to be mimicked. In this regard, we initiated the development of anthropomorphic tissue-mimicking mockups (TMM) that exhibit the major mechanical, anatomical and pathological characteristics of vessels. The TMM is made of a cryogel, polyvinyl alcohol cryogel (PVA-C), which has excellent biocompatibility and is suitable for imaging modalities. By varying the parameters during cryogel fabrication, it possible to tailor the mechanical strength of PVA-C to that of human arteries. The project aims particularly at developing a dedicated CAM to activate the phantom and reproduce the physiological displacements of the myocardium wall during heart beat contractions.
12—Development of a Natural Energy Powered Ventilator
Client
rosaire.mongrain [at] mcgill.ca (Prof. Rosaire Mongrain)
Project
The project consists of designing a low-cost yet efficient mechanical ventilator for use in localities where modern conditions of steady reliable electric grid is not available. The ventilator must be compact and must not require electricity. The device should utilize any energy source readily available such as human power, water current, wind, etc. The main challenge is to design the product with high medical standards while maintaining a flexible range of operation to minimize adverse effects of mechanical ventilation. The technology aims at combining Zeolite materials to enhance O2 concentration and exploit the ventilator rotor concept to generate the needed conception. The Capstone project aims at developing further concepts, optimizing and testing the design for the target operating regime (0-40 cmH2O, up to 1000 ml, respiratory rate 4-45 bpm, flow rates 0-100 lpm). The main objective is to design parts of the new ventilator, assemble and test the ventilator efficiency.
13—Design of a Drug-Eluting Coating for Vascular Technology using Carbon Nanotubes
Client
rosaire.mongrain [at] mcgill.ca (Prof. Rosaire Mongrain)
Project
Implanted medical devices (stents, heart valves, heart pump[s) are usually coated for releasing medical compounds to control thrombogenesis (blood clots) and inflammation. Current coatings technologies rely on polymer carrier (porous or in solution). These are associated with limitations (toxicity, carrying capacity) which restrict its use to certain conditions.
We developed a new paradigm for drug elution based on carbon nanotubes (CNTs). The concept is to generate a controlled density and intertwined structure of CNTs to achieve entrapment of the chemical compound (in analogy to a carpet structure). Preliminary results have shown the potential of the concept for controlled retention and release of a drug compound.
The objective is to design a testing rig to assess the drug elution from the nano-coating. This needs to reproduce the artery flow flow conditions and allow for fluid sampling for the concentration analysis.
14—Addition of Abdominal Muscles into a Robotic Spine
Client
mark.driscoll [at] mcgill.ca (Prof. Mark Driscoll)
Project
In the Musculoskeletal Biomechanics Research Lab (MD 163) there is a robotic spine which is driven by pneumatic muscle contractions. In brief, an air compressor fills the muscle bladders imparting controlled contraction of select muscles related to spine. In turn, this moves and controls the position of the robotic spine upon which we can conduct experiments. However, the robotic spine is missing a “six pack” (muscles not …) while its abdominal region is present. The role of the Capstone team will be to figure out how to include the presence of abdominal muscles (rectus and transverse abdominus as well as internal and external obliques) into the robotic spine. Ideally, they should have an active element to them in order to control contractions. They may also be adjustably passive. The Capstone team are encouraged to dream up any solution they come up with! Specifically, the team are expected to study the problem at hand, propose their own solution, build it, and then test it. Many solutions are feasible and the team of the Musculoskeletal Biomechanics Research Lab look forward to working with the selected group.


15—Safety Assessment of Robotic Spine Set-Up
Client
mark.driscoll [at] mcgill.ca (Prof. Mark Driscoll)
Project
In research, safety should always be a forefront consideration. In the Musculoskeletal Biomechanics Research Lab (MD 163) there is a robotic spine which is driven by pneumatic muscle contractions. In brief, an air compressor fills the muscle bladders imparting controlled contraction of select muscles related to spine. In turn, this moves and controls the position of the robotic spine upon which we can conduct experiments. The Capstone project will consist of assessing the current experimental set up in order to make safety recommendations should the system ever fail. That is the project consists of interpreting, proposing, developing, and testing a failsafe system in place. The Capstone team are encouraged to think outside the box. For example, the solution could be an accessible enclosure which only allows experiments to be conducted when secured. Many alternatives exist. The team will be responsible for determining the specifications and will be given independence towards what solution they converge on to best meet the clients “want”.
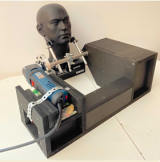
16—Oscillating Device for Postural Correction of Temporomandibular Joint (TMJ) Disorders and Obstructive Sleep Apnea
Client
natalie.reznikov [at] mcgill.ca (Prof. Natalie Reznikov)
Project
This oscillating device is a physiotherapy appliance for clinical conditions having abnormal muscular tone in the face and neck region and a habitual (acquired) abnormal position of the lower jaw (mandible) and neck. The first prototype has been designed and assembled by a Capstone team in 2020-2021. This is the the second iteration of the project where we expect to improve the performance and physical characteristics of the device.
This biomedical device lowers the muscular tone of the craniofacial complex by applying mechanical vibrations in the range 100-300 Hz. Such vibrations induce relief in habitual muscular tone and thus alleviate posteriorly misplaced (retrognathic) occlusion of the mandible, and clenching of teeth. When the mandible regains its physiologic position where teeth are normally out of contact at rest, the backwards displacement of the tongue and the pharynx is also expected to diminish – thus improving breathing. It is expected that applying vibration in short bouts will alleviate dental clenching, temporomandibular joint pain and dysfunction, obstructive sleep apnea, certain varieties of neck pain, and will improve head posture and facial appearance in the subject.
Expected improvements of the design:
- alternative source of vibrations within approximately the same range of frequency and amplitude;
- weight reduction;
- noise reduction;
- anatomically accurate design of contact parts;
- implementation of safety measures;
- aesthetically gratifying layout.
The final design should be suitable for clinical trials on healthy and affected volunteers.
Current appearance of the device:
17—Epipen Redesign
Clients
moshe.ben-shoshan [at] mcgill.ca (Dr. Moshe Ben-Shoshan) and mark.driscoll [at] mcgill.ca (Prof. Mark Driscoll)
Project
Current auto deployment epinephrine devices are bulky and expensive. These devices must be carried by those with severe allergies in order to halt an anaphylaxis reaction, should one occur. These devises comprise a fixed dose of epinephrine, a pre-loaded mechanism to deploy a needle of fixed length, and a means to deliver the epinephrine through the needle when deployed. Many other design solutions present feasible alternatives to the conventional designs used in market today. The role of the present design project would be to miniaturize the above design while maintaining the same reliable outcome. Furthermore, a means of auto emergency notifications should also be integrated into the design.
18—Accurate Dosing for Oral Immunotherapy
Clients
moshe.ben-shoshan [at] mcgill.ca (Dr. Moshe Ben-Shoshan) and mark.driscoll [at] mcgill.ca (Prof. Mark Driscoll)
Project
Food allergies are very prevalent in Canada with over 3 million know cases while anaphylaxis is increasing annually. This poses a particular challenge both in management and treatment. Over the last decade a trend in deterrent treatments has been adopted where allergens, in controlled amounts, are given to the patient with the goal of desensitization. This is known as oral immunotherapy (OIT). The challenge with this process is the ability to provide controlled amounts of the allergen to the patient. More specifically, for example, only [0.03-0.1] mg of egg or milk protein can elicit a reaction. This provides a target desensitization of at least multiple times these amounts to offer protection, with a safety factor, when considering accidents or cross contaminations. Thus, it is the objective of the capstone group to devise a means to enable accurate dossing of common allergens with the aspiration of facilitating and encouraging more widespread practice of OIT.
19—Design and Manufacture of a Prototype 3D Bioprinting Device
Client
showan.nazhat [at] mcgill.ca (Prof. Showan Nazhat)
Project
The aim of this MEDTEC capstone design project is to build a prototype biofabrication/3D bioprinting instrument based on a McGill-led technology, gel aspiration-ejection (GAE). GAE has been demonstrated to be highly effective in generating tissue-like bioinks based on fibrillar collagen and other proteins. In the GAE approach, precursor isotropic hydrogels, prefabricated from a range of collagen concentrations, are aspirated into a capillary through the application of negative pressure, thereby simultaneously inducing both compaction and mesoscale anisotropy on the hydrogel. This is facilitated by aspirating the fibrillar collagen component of the hydrogel into the capillary thereby expelling the excess casting fluid used in the collagen self-assembly process. By subsequent reversal of the pressure, dense collagen gels can be controllably ejected. Precise bioink properties can be predicted through a mathematical compaction factor whereby collagen bioinks with modular density and anisotropy, seeded cell density and temporal functionality can be modelled and biofabricated.
The capstone project team will ideally be composed of four highly motivated engineering students, one student each from Mechanical Engineering, Software Engineering, Electrical Engineering, and Bioengineering to collaborate on designing and building the prototype instrument.
20—Design and Prototyping of a Dynamic Range of Motion Assessment Tool for the Shoulder
Clients
carl.laverdiere [at] mail.mcgill.ca (Carl Laverdière), Orthopaedic Surgery resident (main contact person) paul.martineau [at] mcgill.ca (Dr. Paul Martineau), Orthopaedic Surgeon
Project
It is clinically challenging to measure the range of motion of patient’s limbs consistently between observers. At the moment, the two best tools to assess range of motion while following a patient is a goniometer (pretty archaic, look it up) or eyeballing (obviously inaccurate).
Thus, the objective of this project is to design an apparatus capable of quantifying and tracking the range of motion of the arm from the shoulder joint quickly and accurately. The characteristics needed from the clients are quantitative data in 3 dimensions (x,y,z), a graphical representation of this data as well as a mean to compare with previous tests performed by the same patient. The design team is welcome to brainstorm on potential solution, however they need to keep in mind the portability, ease of use in the clinical setting as well as cost.
The aim is for this device to be used in the orthopaedics clinic to quantitively assess the patients pre-operatively, post-operatively and throughout their rehabilitation process to help the patients get better.
21—Design of Intervertebral Disc Bioreactor with Precise Complex Loadings
Client
jianyu.li [at] mcgill.ca (Dr. Jianyu Li), Lab of Biomaterials Engineering, Faculty of Engineering
Project
Damage of Intervertebral discs (IVDs) have been proven to cause lower back pain. One of the major causes of IVD damages is the complex mechanical loadings experienced during daily activities. This complex loading includes axial compression, torsion, flexion, extension, and lateral bending. To understand the effect of complex mechanical loadings to IVD damage and develop effective treatments, ex vivo culturing of the entire disc organ using bioreactors are in high demand. Though the effect of static and dynamic axial compression has been studied extensively, little is known about the biomechanical response of IVD under the condition of dynamic complex loadings. Due to this fact, it is necessary to develop an IVD bioreactor capable of applying these complex load cases. This project aims to develop a new organ culture loading system with high loading accuracy and resolution. The loading system is expected to consist closed-loop control with real-time load and displacement readouts. The culture system is expected to have the ability to accommodate for both human and bovine IVDs. It is necessary to be biocompatible and include culture media and gas exchange systems. The performance of this bioreactor will be further validated in parallel ex vivo studies of bovine IVD and will be later incorporated with cell-laden hydrogels to study the effects of dynamic biomechanical environments on cellular functions.
22—An In-Vitro Testing Platform for Evaluating the Sealing Performance of Adhesive Sealants
Client
jianyu.li [at] mcgill.ca (Dr. Jianyu Li), Department of Mechanical Engineering and Department of Biomedical Engineering, Faculty of Engineering
Project
Wound closure is a fundamental and practically important problem, underpinning many health issues such as hemorrhage, which account for 10% of death globally, thus calling for strong and robust adhesive sealants. The commercially available tissue sealants such as TISSEEL, COSEAL and DURASEAL are commonly used for halting the surgical bleeding and closing the wound. Naturally, human body produces biological sealants such as blood clot, which also plays an important role in hemostasis and wound healing. The cohesion and adhesion energies are important metrics for evaluating the sealing performances of the above-mentioned adhesive sealants. However, due to their ultra-soft and brittle nature, the conventional testing specimen for measuring adhesion energy, such as the peeling test, is not applicable. It is therefore desired to design a novel testing apparatus which can accurately measure the adhesion/cohesion energies of the sealants regardless of their softness and brittleness. The project requires four undergraduate students to design and prototype a testing platform which can be used to measure the adhesion energy of the adhesive sealants. Briefly, the testing platform includes bulging up a thin film of adhesive sealant by pumping in liquid and using cameras to capture the bulging profile, from which the adhesion energy of the adhesive can be estimated. The tasks include the design of the testing rig, 3D printing and assembly of the parts and camera calibration, and finally the validation test. The students will have the chance to work with mechanical engineers, bioengineers, chemists, biologists, and surgeons.
23—Utilizing Machine Learning Algorithms to Explore Aspects of Surgical Expertise on a VR/AR Surgical Spine Simulator
Clients
Musculoskeletal Biomechanics Research Lab - Professor Mark Driscoll and Sami Alkadri (PhD Student)
Contact
mark.driscoll [at] mcgill.ca (Prof. Mark Driscoll), sami.alkadri [at] mail.mcgill.ca (Sami Alkadri) (PhD student)
Background
Teaching hospitals are realizing the risk of conventional surgical training methods; thus, researchers are exploring the promising results exhibited by virtual reality (VR) pilot training systems for its adaptation to the medical field. The great complexity and high demand of spinal surgeries led to the increased interests in developing novel VR simulators for spinal procedures. Recently, machine learning algorithms are coupled to surgical simulators to give further insights into aspects of the surgical performance that differentiate levels of expertise. Often, deeper subsets of machine learning, such as artificial neural networks (ANNs), might be needed to correctly learn complex non-linear patterns within the given dataset. When combined to virtual reality surgical simulators, the algorithm not only has the potential to correctly predict the different surgical classes, but it can also provide a deeper insight into the impact of the different performance metrics on the classifications. The outlined project is part of the development of a VR/AR surgical training platform to train orthopedic and neurosurgeons in advanced spinal surgery techniques. The platform is developed by McGill University in affiliation with CAE Healthcare and DePuy Synthes (part of Johnson & Johnson Medical Devices).
Scope
Project objectives include developing and employing multiple machine learning algorithms and subsequently compare their performance to the already developed Neural Network Model. Furthermore, explore data augmentation techniques to amplify the small dataset deployed in the project.
Preference
Students with machine learning and computational background is preferred.
24—In-Vivo Low Profile Percutaneous Tissue Homogenizer
Client
louis-martin.boucher [at] mcgill.ca (Prof. Louis-Martin Boucher)
Project
The goal of the project is to be able to generate a self-tumoral vaccine, in-vivo, by homogenizing/mixing an ablated tumor in the liver with an immuno-stimulant (adjuvant). Normally to do this, one would need to excise the tumor, homogenize it with the adjuvant ex-vivo and then use this as a vaccine. However, this demands very special techniques of sterility and tissue processing, not easily available and prohibitively costly.
In interventional radiology, we routinely ablate tumors in the liver, leaving the dead tumor in place. The idea is to try to use the dead tumor left in the liver to stimulate the immune system. This requires some mechanism to mix the ablated tumor in-vivo with the adjuvant. This would be done via a percutaneous access.
The system we are looking for is a low-profile system (that could be engaged through a 15g needle) into the ablated tumor under US guidance. Needle length would have to be approximately 20 cm in length. Once the homogenizer needle is in place it would have to be able to inject a specific volume of immunostimulator (gelified liquid) while mixing/homogenizing this with the ablated tumor. The system would need to be controllable in such a way that a relatively specific volume of tissue is mixed/homogenized so that we do not damage the normal liver around the ablated tumor, whether time based or speed based. Ideally, the system would need to be autoclavable/sterilizable.
We have published the idea in a previous publication. See "Carias et al., Ex Vivo Study of Experimental Method Toward Future In Vivo Tissue Processing for Self-Anti-Tumoral Vaccinations, Cardiovasc Intervent Radiol. 2021 May;44(5):818-821.doi: 10.1007/s00270-020-02736-7. Epub 2021 Jan 27." For this we used a basic thrombectomy device that consists of a rotating wire, but at low speed and which was insufficient to optimally mix the tumor with the gellified liquid. We are therefore at a road block presently and the design of this system will allow us to move forward in developing a technique to use someone's own ablated tumor, in-vivo, to generate a anti-self vaccine, allowing us to create personalized medicine with universal tools, a possible game-changer in the fight against cancer.