What if your significant other gifted you baking powder instead of chocolates? What if they invented baking powder? In 1843, British chemist Alfred Bird did just that for his lucky, yeast-allergic, bread-loving, wife. This same recipe for baking powder is used to this day. But when it comes to baking cookies, muffins or cakes, recipes will call for baking soda, baking powder, or both. Just like their names, these products are very similar, but not exactly the same.
The active ingredient in both baking soda and baking powder is sodium bicarbonate, or NaHCO3. This compound acts as a leavening agent — which means that it reacts to release carbon dioxide gas (CO2) which gives bread, cake, and pancakes that fluffy texture we know and love.
Let’s break down the chemistry. There are two things that can trigger this reaction: heat and acid.
In the presence of heat, sodium bicarbonate undergoes what is called a thermal decomposition reaction, releasing a molecule of CO2 for every two molecules of sodium bicarbonate. This occurs at a minimum temperature of around 80°C or 180°F. Since recipes generally require a minimum temperature of 300°F, we’re well above the threshold for this reaction to occur.
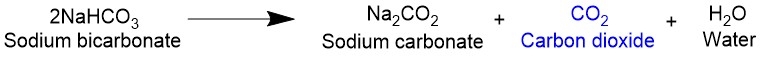
In the presence of an acid — which can be in the form of lemon juice, buttermilk, or cream of tartar — sodium bicarbonate undergoes an acid-base reaction. In this case, a molecule of CO2 is released in a 1:1 ratio with sodium bicarbonate. That means that you need half the amount of active ingredient to get the same amount of fluffiness as the previous reaction. An acid-base reaction is preferred in order to maximize the carbon dioxide produced.

So, what’s the difference between baking soda and baking powder? Baking soda is pure sodium bicarbonate. Recipes that already have some sort of acid, buttermilk for example, will generally use baking soda. Baking powder is sodium bicarbonate combined with a powdered acid; it is pre-packaged to react in the presence of moisture and heat. Recipes that don’t already include an acidic component will generally use baking powder. Sometimes both these ingredients are included because some of the acidic component needs to be left behind for taste, for example citric acid from lemon juice.
Since baking soda and baking powder contain the same active ingredient, you can substitute one for the other in your recipes, with some adjustments. Baking soda needs to be supplemented with the addition of an acid, such as cream of tartar, to produce the carbon dioxide gas. Baking powder contains less sodium bicarbonate per a certain volume compared to baking soda, so you would need to scale up the amount of baking powder used. Both substitutions might alter taste and texture slightly depending on the recipe but will work in pinch in order to get the rise in your dough.
Cat Wang recently graduated from McGill University with a Bachelor of Science (BSc) degree in the anatomy and cell biology program and is currently deciding on a Master’s program.







