Imaging Purkinje cell axonal torpedoes
By: Connie H. Li
Supervisor: Professor Alanna J. Watt
Acknowledgements: Daneck Lang-Ouellette, Ph.D. Candidate

With long axons1 and elaborate dendritic branching1,2 like boughs of a tree, Purkinje cells of the cerebellum are distinguished by their idiosyncratic morphology3. As the sole output neurons of the cerebellar cortex4,5, a region that regulates motor learning6 and coordination7-9, Purkinje cells play a vital role in relaying information about movement. Since this information is propagated by the axons of Purkinje cells10, axonal morphology is a crucial consideration in the study of cerebellar development and disease.
In disorders such as ataxias11-13, essential tremor14,15, and other neurological conditions like Parkinson’s disease16, swellings in Purkinje cell axons have been observed. As such, these focal axonal swellings17—also termed “torpedoes”18—were thought to be a hallmark of neurodegeneration13,19. However, torpedoes have also been identified in the developing cerebellum19,20 and, in these cases, they are unlikely to coincide with elimination processes like axonal pruning19. In fact, torpedoes could constitute a compensatory mechanism that responds to neuronal firing gone awry. Findings in the Watt laboratory have shown that Purkinje cell axons with torpedoes are significantly less likely to exhibit failures in action potential propagation compared to Purkinje cell axons without torpedoes (Lang-Ouellette, unpublished). Indeed, this observation has led our lab to propose that torpedoes may be acting homeostatically, enabling Purkinje cells to improve in firing fidelity. Given this supposed homeostatic function, all Purkinje cells should generate torpedoes to sustain optimal firing. Yet, torpedo formation only occurs on select axons: in the developing mouse cerebellum, the proportion of Purkinje cells with torpedoes peaks at 40% during postnatal day 1119. It is unclear why the remaining Purkinje cells do not form torpedoes.
To probe potential mechanisms that contribute to torpedo formation, I undertook the investigation of Purkinje cell axonal morphology. While the physiological changes due to torpedo formation have been well described (Lang-Ouellette, unpublished), the physical changes, including magnitude and rate of growth, are not fully understood. Since torpedoes are broadly defined as marked swellings19,21, I sought to delineate the morphology and dynamics of torpedo growth. We hypothesized that axons exploit existing matter for torpedo formation; ergo, axons with segments of greater volume may be more likely to develop torpedoes. If there is a morphological “signature” that could differentiate between torpedo-forming and non-torpedo-forming Purkinje cells, we could harness this predictive power to understand if cerebella with more torpedoes have a heightened resilience to movement-impairing diseases.
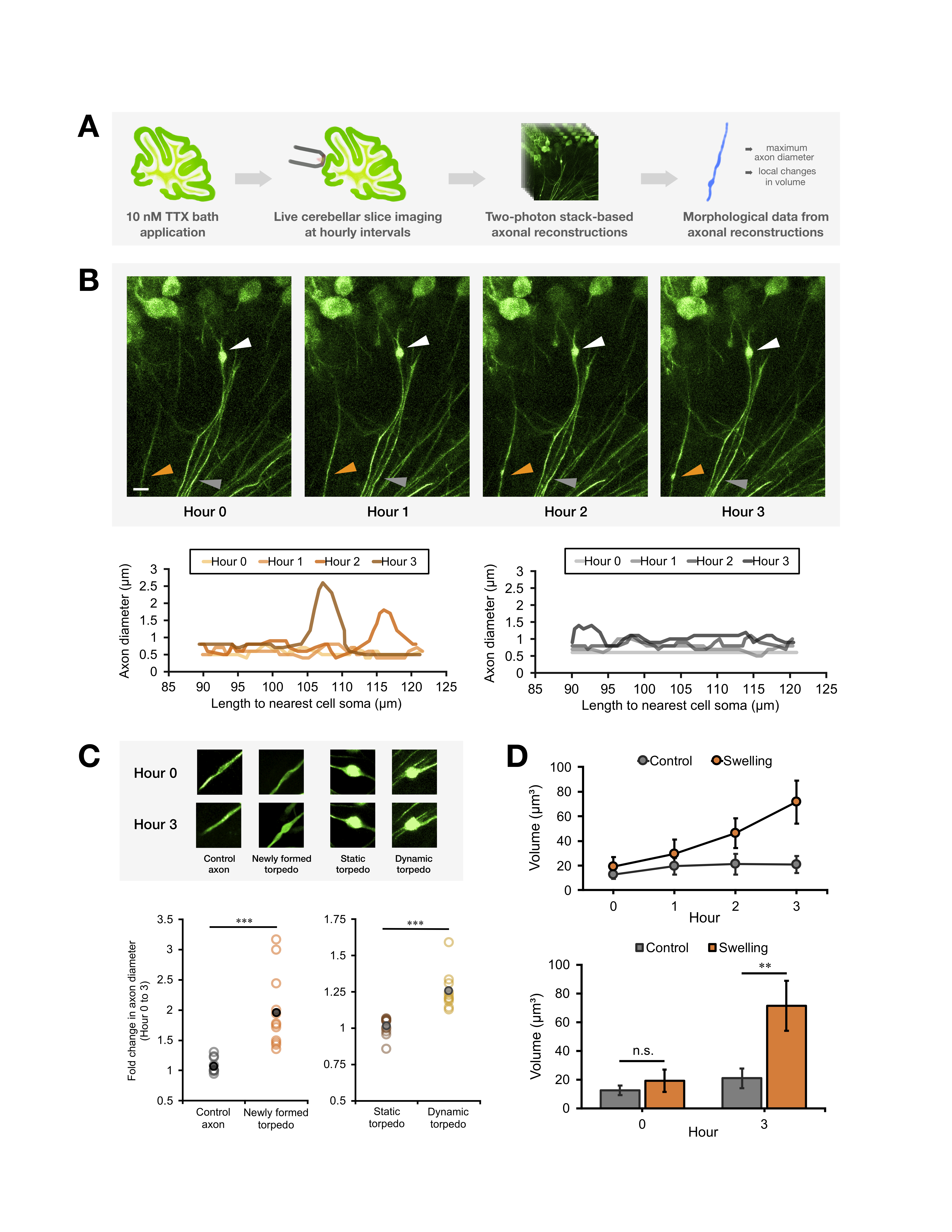
In order to visualize torpedoes, our lab employs an original protocol to model torpedo development in mice. Live slices are procured from the cerebellar vermis of L7-tau-GFP mice (male and female). This transgenic construct labels Purkinje cells with green fluorescent protein (GFP)22. To incite torpedo formation, a bath containing 10 nM of tetrodotoxin (TTX) is applied to each slice. At this concentration, TTX blocks a fraction of all sodium channels in Purkinje cells. This mimics failures in action potential propagation and subsequently triggers torpedo formation (Lang-Ouellette, unpublished). By simultaneously applying this bath and imaging the slices with two-photon microscopy, torpedo growth can be recorded at hourly intervals. With these time-lapse images as a template, I manually designed axonal reconstructions in Neurolucida, a tool for 3-dimensional modelling of histological samples23. Undertaking these comprehensive morphological analyses allowed micron-level fluctuations in axon diameter and volume to be recorded.
Imaging Purkinje cells at hourly intervals captured seemingly negligible changes in axonal morphology. The magnitude of change distinguished torpedo-forming axons from non-torpedo-forming axons (i.e. “control axons”). As predicted, torpedoes do not form on all axons in a given vicinity. Some axons do not change over time, maintaining a thin and uniform axon diameter (Figure 1B, grey arrow). Meanwhile, some axons seem to be a “control axon,” but later produce a swelling over three hours (Figure 1B, orange arrow). In contrast, other swellings are fully formed prior to imaging, and could be dubbed, “static torpedoes,” which do not grow over time (Figure 1B, white arrow). Quantifying diameter changes led to a more nuanced understanding of the magnitude of growth. While control axons have an axon diameter of 0.5 to 1.5 µm, torpedo-forming axons can swell beyond 4 µm, and also have a wider range of fluctuation.
Upon inspection of all torpedo-forming axons, there seemed to be subsets with differential magnitudes of change. For instance, as characterized in Figure 1B, some axons look like a “control axon” at Hour 0, but develop a swelling by Hour 3. Such axons could be considered to have “newly formed torpedoes” (Figure 1C). On the other hand, other torpedoes can maintain the same diameter over time—“static torpedoes”—or continue to increase in diameter—“dynamic torpedoes” (Figure 1C). Although these categories are artificially derived from morphological analyses, they can inform our understanding of the specificity of torpedo formation. Ultimately, illustrating torpedo dynamics has uncovered potential points in the “life cycle” of axonal swellings.
To better understand why some axons displayed a newly formed torpedo (i.e. “swelling”) while others did not, volumetric changes were investigated. We predicted that initial volume in axons with swellings would be greater than control axons; however, the results proved to be contrary. At Hour 0, there are no significant differences in the volume of an axon segment that will form a torpedo versus one that will not (Figure 1D). Yet, over time, axons with swellings greatly increase in volume. This unprecedented finding suggests that, even with precise volumetric analyses, it is not possible to predict if an axon will develop a torpedo.
Future experimentation will involve probing the mechanisms that cause volumetric increases during torpedo formation. To shed light on sub-cellular structures that may contribute to swelling, electron microscopy will be used to image of the internal milieu of torpedoes. By seeking richer descriptions of torpedoes’ morphology and dynamics, we can harness their association with improved firing fidelity to understand developmental and pathological states of the cerebellum.
Click image for larger version.
Figure 1. Axonal reconstructions based on time-lapse images acquired with two-photon microscopy enable the modelling of differential changes in Purkinje cell morphology.
(A) Schematic diagram depicting the induction and imaging of torpedo growth. A 10 nM bath of tetrodotoxin (TTX) is sufficient to block a proportion of sodium channels in Purkinje cells. This disrupts action potential propagation and leads to torpedo formation, which can be imaged with two-photon microscopy at hourly intervals. The image stacks serve as templates for axonal reconstructions. (B) (Top) Representative images of lobule III of the cerebellar vermis of L7-tau-GFP mice. Scale bar = 10 µm. Each image is a maximal intensity projection with a depth of 20 µm. Points of interest are indicated by arrows: orange arrow highlights an axon that appears relatively thin at Hour 0 but later produces a torpedo; grey arrow indicates a “control axon” with a diameter that remains stable over time; white arrow points to a torpedo that has already formed a torpedo by the time of imaging and also serves as a control. (Bottom left) Precise fluctuations in axon diameter over time, corresponding to orange arrow. (Bottom right) Precise fluctuations in axon diameter over time, corresponding to grey arrow. (C) (Top) Comparing slices imaged at a certain time point (i.e. Hour 0) with the final time point (i.e. Hour 3) reveals differential changes in maximum axon diameter. Based on the fold change in maximum axon diameter, Purkinje cell axons can be clustered into one of four broad categories. (Bottom left) Control axon: n = 12; Newly formed torpedo: n = 12. *** signifies p = 0.00031. (Bottom right) Static torpedo: n = 12; Dynamic torpedo: n = 10. *** signifies p = 0.000191347. Statistics were conducted with an unequal variance t-test. (D) (Top) Axonal reconstructions capture changes in volume over time. 20 µm segments were taken from control axons and axons with newly formed torpedoes (i.e. “swellings”). The segments’ midpoint were aligned to the position at the maximum axon diameter. (Bottom) Comparing absolute volume of control axons and axons with swellings does not demonstrate a significant difference at Hour 0. By Hour 3, axons with swellings have a significantly greater volume than control axons. Control and swelling groups: n = 8 axons from 4 mice. ** signifies p = 0.02414535. Statistics were conducted with an unequal variance t-test. Error bars: standard error of mean.
References
- Purkinje, J. Über Neuesten Untersuchungen aus der Nerven- und Hirn-Anatomie. (Gottlieb Haase, 1838).
- Ramón y Cajal, S. Histologie du système nerveux de l'homme et des vertébrés. (Maloine, 1911).
- Poulain, F. E. et al. SCLIP is crucial for the formation and development of the Purkinje cell dendritic arbor. J Neurosci 28, 7387-7398, doi:10.1523/JNEUROSCI.1942-08.2008 (2008).
- Eccles, J. C. The cerebellum as a neuronal machine. (Springer, 1967).
- Dusart, I. & Flamant, F. Profound morphological and functional changes of rodent Purkinje cells between the first and the second postnatal weeks: a metamorphosis? Front Neuroanat 6, 11, doi:10.3389/fnana.2012.00011 (2012).
- De Zeeuw, C. I. & Ten Brinke, M. M. Motor Learning and the Cerebellum. Cold Spring Harb Perspect Biol 7, a021683, doi:10.1101/cshperspect.a021683 (2015).
- Rolando, L. Saggio sopra la vera struttura del cervello dell'uomo e degli animali e sopra le funzioni del sistema nervoso. (Arnoldi Forni Editore, 1809).
- Holmes, G. The cerebellum of man. Brain 62, 1-30, doi:10.1093/brain/62.1.1 (1939).
- Moberget, T. & Ivry, R. B. Cerebellar contributions to motor control and language comprehension: searching for common computational principles. Ann N Y Acad Sci 1369, 154-171, doi:10.1111/nyas.13094 (2016).
- Ovsepian, S. V. & Friel, D. D. Enhanced synaptic inhibition disrupts the efferent code of cerebellar Purkinje neurons in leaner Cav2.1 Ca 2+ channel mutant mice. Cerebellum 11, 666-680, doi:10.1007/s12311-010-0210-9 (2012).
- Sasaki, H. et al. Neuropathological and molecular studies of spinocerebellar ataxia type 6 (SCA6). Acta Neuropathologica 95, 199-204, doi:10.1007/s004010050787 (1998).
- Yang, Q. et al. Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathologica 100, 0371-0376 (2000).
- Louis, E. D., Kuo, S.-H., Vonsattel, J.-P. G. & Faust, P. L. Torpedo Formation and Purkinje Cell Loss: Modeling their Relationship in Cerebellar Disease. The Cerebellum 13, 433-439, doi:10.1007/s12311-014-0556-5 (2014).
- Louis, E. D. et al. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum 10, 812-819, doi:10.1007/s12311-011-0291-0 (2011).
- Louis, E. D., Lee, M., Cortes, E., Vonsattel, J. P. & Faust, P. L. Matching asymmetry of tremor with asymmetry of postmortem cerebellar hemispheric changes in essential tremor. Cerebellum 13, 462-470, doi:10.1007/s12311-014-0560-9 (2014).
- Louis, E. D. et al. Torpedoes in Parkinson's disease, Alzheimer's disease, essential tremor, and control brains. Mov Disord 24, 1600-1605, doi:10.1002/mds.22567 (2009).
- Ramón y Cajal, S., DeFelipe, J. & Jones, E. G. Cajal's Degeneration and Regeneration of the Nervous System. (Oxford University Press, 1991).
- Bouman, L. Die Histopathologie Der Psychosen. (Springer, 1918).
- Ljungberg, L. et al. Transient Developmental Purkinje Cell Axonal Torpedoes in Healthy and Ataxic Mouse Cerebellum. Front Cell Neurosci 10, 248, doi:10.3389/fncel.2016.00248 (2016).
- Gravel, C., Leclerc, N., Plioplys, A. & Hawkes, R. B. Focal axonal swellings in rat cerebellar Purkinje cells during normal development. Brain Res 363, 325-332 (1986).
- Louis, E. D., Yi, H., Erickson-Davis, C., Vonsattel, J. P. & Faust, P. L. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett 450, 287-291, doi:10.1016/ j.neulet.2008.11.043 (2009).
- Watt, A. J. et al. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci 12, 463-473, doi:10.1038/nn.2285 (2009).
- Nedelescu, H. & Abdelhack, M. Comparative morphology of dendritic arbors in populations of Purkinje cells in mouse sulcus and apex. Neural Plast 2013, 948587, doi: 10.1155/2013/948587 (2013).