Reactions of atmospheric oxidants with volatile and semi-volatile compounds and intermediates: Kinetic, thermo-chemical and mechanistic studies
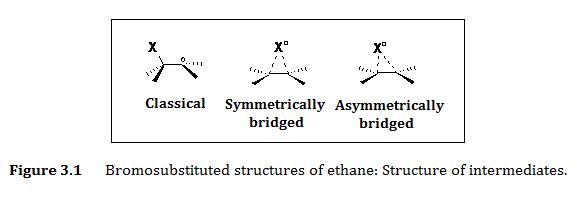
The evidence for halogens such as Cl-atom and Br-atom (and their oxides) reactions, in addition to HO, O3, NO3, rapidly became of key interest to many researchers. These radicals can react rapidly with atmospheric compounds, playing key roles in the degradation and transformation of various chemicals in the troposphere. Br-atoms have also been found to be major players in the destruction of unsaturated compounds and one of the most important greenhouse gases, ozone. Many of these primary and secondary products are toxic to plants and animals, and it is essential that the atmospheric fate of these molecules is determined. The radical addition reactions to carbon-carbon double bonds have been a topic of long-standing interest to us. These reactions normally lead to the formation of carbon-centered radicals, as the π-bond upon interaction with unpaired valence of the approaching radical transforms into new σ-bond and the radical centers at the carbon atom (Figures 1.3 and 1.4). If the radical attacking the alkene is a halogen atom, the addition reaction gives a b--haloaklyl radical. In addition to halogen-alkene reactions, b-haloaklyl radicals can also be formed in radical halogenation of (halo)alkanes, as well as in the thermolysis or photolysis of various halogenated compounds. The importance of b-halolakyl radicals in many chemical processes led to a number of experimental and theoretical studies of their structure and reactivity. The 1,2-halogen migration and its role in the stereoselectivity of the halogenation reactions of various alkanes and alkenes has been given special attention. In order to interpret surprisingly high steroselectivity of the reactions of bromo-substituted radicals, several studies (Goering et al., Thaler, and later Skell and Traynham) proposed, in addition to the classical carbon-centered b-halolakyl radical structure, an alternative bridged structure:
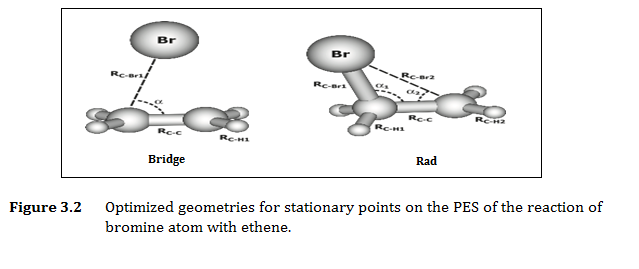
We combine experimental and computational study of the reactions of alkenes with halognes such as bromine atoms.. Effective rate constants k of the gas-phase reactions of several alkenes with halogens at tropospheric conditions are measured and used in combination with the literature data to find a quantitative structure-activity relationship (QSAR) between the lnk and the HOMO energy of the alkene, which holds for all alkenes studied. We have found this method is appropriate except for tri- and tetrachloroethene. To gain insight into possible sources of this irregularity, high-level CASPT2 (including spin-orbit coupling effects) and DFT calculations of structures and stability of bromine atom adducts with ethene and tetrachloroethene have been performed in our labs. We presently investigating the role of stable structure, as well bridged structures to gain insides on pre-reaction, which might exist as a local minimum on the potential energy surface. We also strive to understand the mechanistic differences amongst the reactions of halogenated alkenes.
Selected related publications in this domain
- A. Garib, Q, Timergazin, and P.A. Ariya, "Kinetics and product studies of Cl-atom initiated of selected hydrocarbons in the marine boundary layer", Canadian Journal of Chemistry, 84, 1686-1695 (2006)
- Qadir Timerqazin and Parisa A. Ariya, "Kinetics and mechanistic studies of Cl-atom initated reaction of terpenes", Physical Chemistry-Chemical Physics, 3, 3981-3986 (2001)
- Sandrine Coquet and Parisa Ariya, The temperature dependence of Cl-atom initiated reactions of selected alkenes under tropospheric conditions, International Journal of Chemical Kinetics, 32, 478-488 (2000)
- V. Catoire, P. A. Ariya, H. Niki and G. W. Harris, FTIR study of Cl and Br atom oxidation of trichloroethylene at T = 296?2 K;, International Journal of Chemical Kinetics, 29, 695-704 (1997)
- J. Chen, V. Young, P .A. Ariya (Hooshiyar), M. Hurley and H. Niki, FTIR study of the Cl-initiated oxidation of ethylene oxide, J. Phys. Chem, 99, 4071-4077 (1995).
- Parisa A. Ariya (Hooshiyar) and Hiromi Niki, Reaction rate of Cl-atoms with a series of C2-C8 alkanes at T = 296 +/- 2 K, Int. J. Chem. Kinetics, 27, 1197-1206 (1995).