
Associate ProfessorDirector, School of Human NutritionT: 514-398-2515 | ryan.mailloux [at] mcgill.ca (Email) | Macdonald-Stewart Bldg MS2-042a |
Degrees
-
BSc (Honours) - Biochemistry/Laurentian University
-
PhD - Biomolecular Sciences/Laurentian University
Recruiting
Looking for a Postdoctoral Fellow - joint supervision with Professor Luis Agellon.
Having expertise in nutrient metabolism and redox biology would be viewed as an asset.
Short Bio
Professor Ryan J. Mailloux received is PhD in Biomolecular Sciences in 2008 from Laurentian University, after which he conducted his postdoctoral work at the University of Ottawa and Carleton University/Health Canada, respectively. Before joining the School of Human Nutrition at McGill University in 2019, he served as a faculty member (tenure-track) in the Department of Biochemistry at Memorial University of Newfoundland (2015-2019). He is now Director of the School of Human Nutrition at McGill.
Research Interests
His research focuses on understanding how mitochondria generate free radicals like hydrogen peroxide and use these reactive molecules in cell communication. Specifically, his work focuses on mitochondrial hydrogen peroxide in the liver, the redox regulatory mechanisms that control its production, and how controlled mitochondrial hydrogen peroxide production can be used it to maintain optimal liver health. Professor Mailloux’s group is also invested in understanding how defects in mitochondrial hydrogen peroxide production by liver mitochondria can contribute to the manifestation of hepatic diseases like metabolic dysfunction induced fatty liver disease (MALFD) and its progression to more advanced liver disorders like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC). Finally, his team discovered reversible redox modifications like S-glutathionylation and S-nitrosylation serve as a novel mechanism for the regulation of hydrogen peroxide production in liver mitochondria. His team is now collaborating with various groups to target these hydrogen peroxide-generating sites with different therapeutics to prevent and treat MAFLD and HCC.
Professor Mailloux has published 94 articles on the topics of mitochondrial bioenergetics, oxidative eustress and oxidative distress, redox homeodynamics, hydrogen peroxide production and how dysfunctional bioenergetics causes metabolic disorders. More information on his research program and publications can be found here:
SCOPUS: https://www.scopus.com/authid/detail.uri?authorId=8664226100
Webofscience: https://www.webofscience.com/wos/woscc/summary/d8775f2b-6a5b-4adf-9be7-22e24062a89e-e23f6cb0/relevance/1
Google Scholar: https://scholar.google.ca/citations?user=M9XKSHEAAAAJ&hl=en
Current Research
These figures were taken from recent selected publications in Redox Biology, The Journal of Biological Chemistry, and Biochimica et Biophysica Acta (Molecular Cell Research)
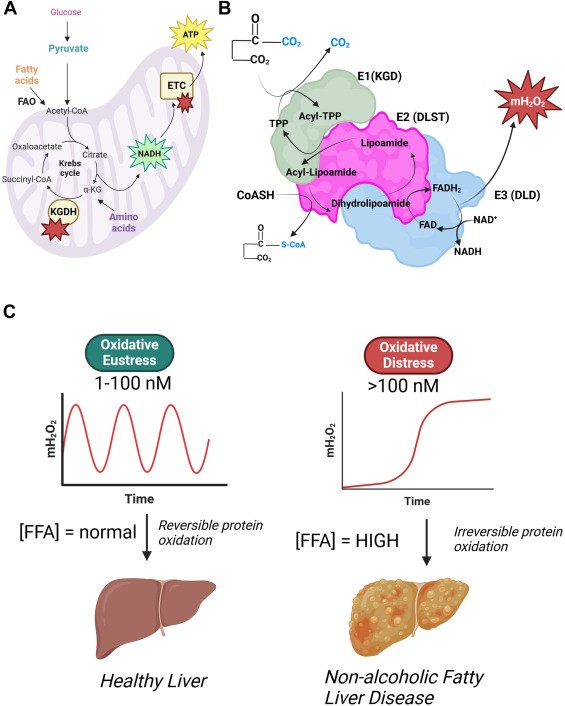
Krebs cycle enzyme alpha-ketoglutarate dehydrogenase (KGDH) in intercellular redox signaling. Professor Mailloux’s group has found is a powerful hydrogen peroxide generator and that is production can be regulated by reversible S-glutathionylation or S-nitrosylation. This can have profound signaling effects in cells and other cells throughout the body. The signals are propagated by hydrogen peroxide, but also through the regulation of different Krebs cycle metabolites, which are also used as messengers in cells.

Redox Biol. 2024 Apr 10;72:103155
Free fatty acids can drive the over production of hydrogen peroxide by alpha-ketoglutarate dehydrogenase, causing oxidative distress leading to MAFLD and MASH, but when production is controlled, the hydrogen peroxide activates oxidative eustress, which is vital for hepatic health.
J Biol Chem. 2024 Mar 11;300(4):107159.
Hydrogen peroxide production by dihydroorotate dehydrogenase and proline dehydrogenase is vital for triggering a hyper-proliferative state in cancer progression. This also forms a vital pyrimidine synthesome platform.

Biochim Biophys Acta Mol Cell Res. 2024 Feb;1871(2):119639.
Cell hydrogen peroxide can be controlled by several feedback loops that modulate its availability, which plays a key role in the propagation of redox signals, or oxidative eustress. Defects in these feedback loops can deregulate the production and availability of hydrogen peroxide, which can cause oxidative distress (commonly referred to as oxidative stress). In cancer cells, these feedback loops are deregulated causing increase hydrogen peroxide and oxidative distress. But, instead of triggering cell cycle arrest and cell death, the cancer cells adapt to the increased hydrogen peroxide and harness it to promote a hyper-proliferative state. This is achieved by shifting the redox stress threshold (RST) through the activation of the transcription factors NRF2, HIF-1alpha, NF-kB, and HSF-1. It is also likely that cancer cells develop increased resistance to so-called “reductive distress”.

Biochim Biophys Acta Mol Cell Res. 2024 Feb;1871(2):119639.
Courses
Publications
To view a list of Dr. Mailloux's publications, click here. Also available on PubMed.

