The internet is full of suggestions that Aspirin can be used to remove stains and get whiter whites. These sites don’t offer any mechanism of this bleaching of course, so I had to try it myself.
Rather than risk my real clothes to this science experiment, I created some stains (tomato and espresso) on pieces of fabric taken from washcloths.
Every site had slightly different instructions, so I chose what seemed like the least nonsense filled options: Reader’s Digest and WikiHow. They both instructed me to soak my fabric in hot water in which I’d dissolved five 325 mg tablets of Aspirin, and leave it to soak. Since I foolishly bought 500 mg Aspirin (it was on sale!) I used 3.25 of my tablets. I ground them up, dissolved them in hot water, and added the fabric.


I also tested a more direct application to give the Aspirin the best chance of working. I ground up another 3.25 tablets, added a bit of water to make a paste, applied that directly to the stains, and let them sit.




While the experiments were brewing, I washed my control stains with ½ cup of bleach (as per the instructions on the bottle) in the washer (on warm and “extra clean” settings).

Once a few hours had passed on the soaking and sitting stains, I washed them in the same way, without bleach, then hung them all on the line to dry. A few hours later I realized it had actually started raining, so I brought them inside and dried them in the dryer.

The waiting in this process gave me some time to do some math.
Brand name Aspirin costs 7 cents per pill, making a single treatment of this method cost only 30 cents.
Pricing other stain removers is a bit more difficult. A rough estimate of the volume of one ‘spray’ from a spray bottle is 0.1 ml. If you used about 5 sprays to cover a stain the price per spot treatment would be about 0.0023 cents. So to make the Aspirin soak worth it, you’d need to be treating more than 13043 stains per load. Perhaps more directly comparable, Oxiclean powder that is added to the laundry costs about 0.17 cents per load.
But, this is a good time to remember that even if the Aspirin is 13000 times more expensive than Shout, we’re talking about pennies here. They’re all dirt cheap. Cost is not the issue with this method. The fact that it didn’t work is.
Neither the soak nor paste method worked as well as good, old-fashioned, bleach.

So why not?
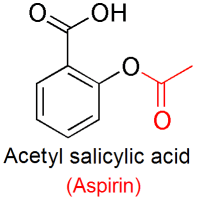
Aspirin is the common name for acetylsalicylic acid (ASA). It’s a prodrug, meaning that the form it’s in while in pills is not the active form of the compound. ASA is absorbed into the bloodstream in the stomach, and is then converted to salicylic acid (SA) (also known as salicylate) in the liver. It’s salicylic acid that disperses through our bodies and causes the pain, inflammation and fever reductions we expect.
SA mainly binds and deactivates cyclooxygenase (COX) enzymes. These enzymes usually produce prostaglandins, pro-inflammatory hormones that help neurons detect pain, and thromboxanes, lipids that promote blood clotting. With fewer prostaglandins and thromboxanes, we feel less pain and blood clots are avoided. That’s why daily low dose aspirin is useful for avoiding heart attacks- it helps prevent the formation of blood clots that could block arteries.
ASA can be broken down by water to form salicylic acid and acetic acid (vinegar). So it could be the acetylsalicylic acid, salicylic acid, acetic acid, or some other Aspirin ingredient that works as a bleaching agent. Bleaches can work either by reducing the molecule that gives something colour (giving it electrons), or by oxidizing it (taking electrons from it).
Luckily, scientists have a measure of how well an agent can reduce or oxidize its subject- reduction potential. The more negative a substance’s reduction potential, the better a reducing agent it is. For example, common bleach (sodium hypochlorite) works by oxidizing stain molecules and has a reduction potential of about +1.2, making it a pretty good oxidizing agent.
Salicylic acid and acetylsalicylic acid both have reduction potentials of about -0.49. Acetic acid breaks down into acetate ions in water and has a reduction potential of about -0.6. Those numbers just aren’t great. The evidence for bleaching properties just isn’t there.
And don’t forget the evidence of tomatoes and coffee that remains on my fabric.
You’re forgiven for believing in the aspirin bleaching method if you, like me, have used a lot of anti-acne products. Many of these products use salicylic acid, benzoyl peroxide, or both, as their active ingredient. If you’ve ever applied a night cream and woken up to a bleached pillowcase, you know that some acne products cause bleaching of fabrics and hair. That’s because, unlike SA, benzoyl peroxide does have a strong bleaching effect, like most other peroxides. The two reagents are used so interchangeably and commonly in skin care products that it’s fairly easy to get them mixed up. Rest assured, a SA only cream won’t wreck your favourite bathrobe.
It also won’t get the lipstick stain out of it.
Want to engage with this content? Comment on this on our Facebook page!







