What to know prior to developing a CPD activity
1. CPD Activity Planning Process Overview
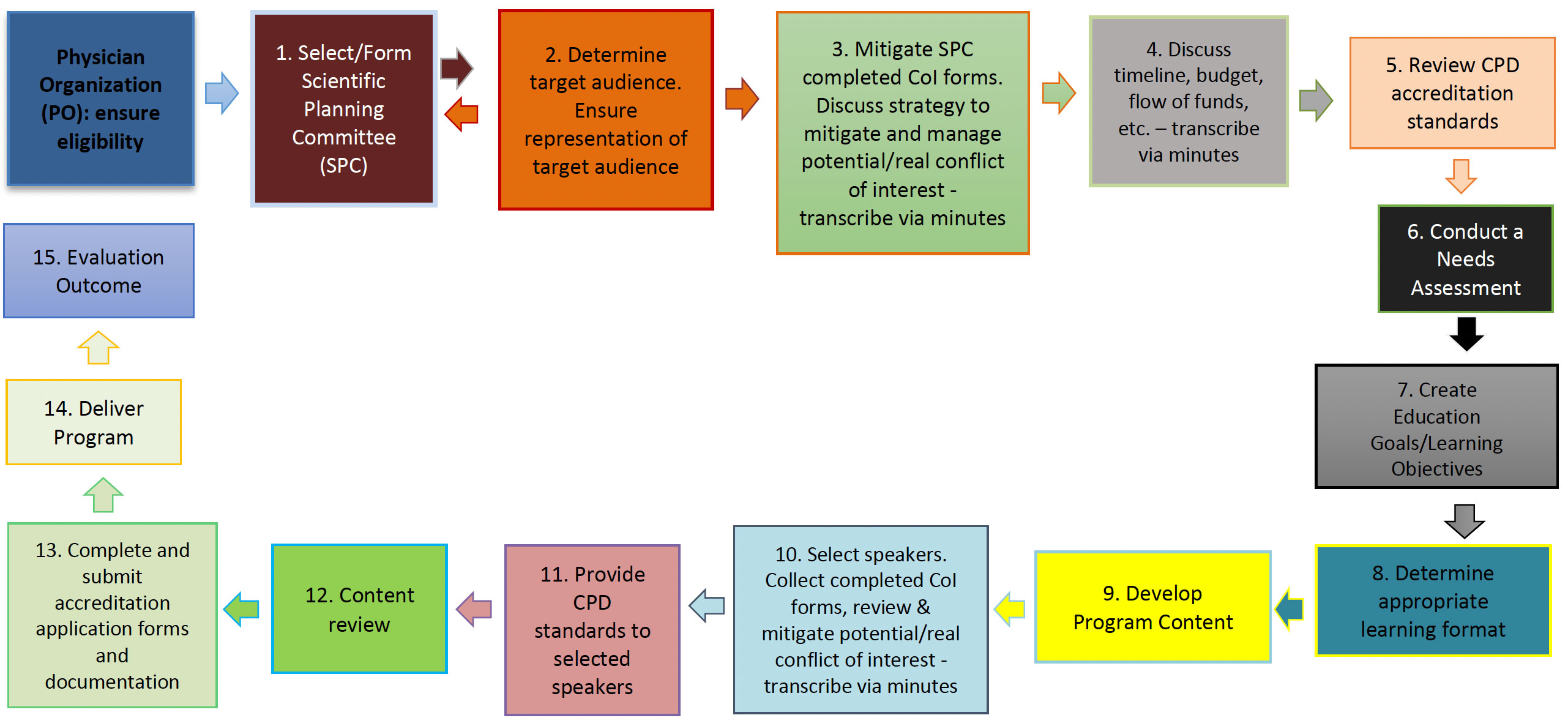
Following the infographic below will ensure the activity is organized according to the ethical, educational, and administrative standards throughout the developing, planning and implementation phases.
In compliance with the National Standard for Support of Accredited CPD Activities, and to safeguard attaining CPD credits for your activity, please ensure to follow the planning process described below:
(Click on the image to enlarge it)
- Select/Form Scientific Planning Committee;
- Determine target audience. Ensure representation of target audience;
- Mitigate SPC completed CoI forms. Discuss strategy to mitigate and manage potential/real conflict of interest - transcribe via minutes;
- Discuss timeline, budget, flow of funds, etc. – transcribe via minutes;
- Review CPD accreditation standards;
- Conduct a needs assessment;
- Create education goals/learning objectives;
- Determine appropriate learning format;
- Develop program content;
- Select speakers. Collect completed CoI forms, review & mitigate potential/real conflict of interest - transcribe via minutes;
- Provide CPD standards to selected speakers;
- Content review;
- Complete and submit accreditation application forms and documentation;
- Deliver program;
- Evaluation Outcome.
CPD activities must be developed or co-developed by a physician organization (PO). The PO must be involved in the development of the CPD activity from selecting the SPC members to the evaluation outcome.
Selection of the SPC must be done with the PO. If developing an activity to be held in Quebec, ensure to engage two committees:
- Scientific Planning Committee;
- Organizing Committee.
The SPC structure must be made up of the target audience. If the target audience is interprofessional, the SPC must include representation from each profession.
The SPC must have exclusive control and assume responsibility over topics, content, presenter selection, and appropriateness of the learning format. As a result, the SPC will review content to ensure the scientific validity and objectivity and certifying that key references are listed on each slide as evidence for claims made.
Prior to developing an activity, the SPC must discuss key elements linked to the development of the activity. Discussion must include: the method used to review the completed conflict of interest forms from all resource persons, including those of the SPC members; strategy used to manage potential or real conflicts of interest; timeline, budget, and the flow of funds; identification and development of the learning objectives; selection of the learning format; selection of speakers, etc.
The SPC must ensure that funds received are in the form of an educational grant payable to the SPC/physician organization and must assume responsibility for the disbursements of funds. Ensuring that there will be no industry influence over any of the planning aspects linked to the activity.
The SPC must conduct a needs assessment.
The SPC is responsible to ensure that content presented avoids copying images, videos, tables, cartoons, and graphs from copyright publications.
The SPC must ensure that all resource persons, including the SPC members, communicate (verbal and with slides) a statement on conflict of interest to the audience.
2. Submitting the Accreditation/Certification Package and Supporting Documentation
The Chair of the SPC is responsible for the signing and submitting the CPD Accreditation/Certification Application Form and submitting mandated supporting documentation to the Continuing Professional Development Office (CPD).
3. Submitting the Post-Program Documentation
No later than eight (8) weeks following the completion of the activity, the Chair of the SPC will complete a final report to be submitted to the Continuing Professional Development Office (CPD). The completed Final Report Form and all supporting materials are mandated to finalize the accreditation/ certification process.
The SPC is responsible for conducting evaluations for individual sessions and the overall program.